Radixin: Roles in the Nervous System and Beyond
- PMID: 39457653
- PMCID: PMC11504607
- DOI: 10.3390/biomedicines12102341
Radixin: Roles in the Nervous System and Beyond
Abstract
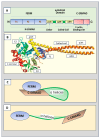
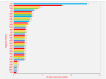
Background: Radixin is an ERM family protein that includes radixin, moesin, and ezrin. The importance of ERM family proteins has been attracting more attention, and studies on the roles of ERM in biological function and the pathogenesis of some diseases are accumulating. In particular, we have found that radixin is the most dramatically changed ERM protein in elevated glucose-treated Schwann cells.
Method: We systemically review the literature on ERM, radixin in focus, and update the roles of radixin in regulating cell morphology, interaction, and cell signaling pathways. The potential of radixin as a therapeutic target in neurodegenerative diseases and cancer was also discussed.
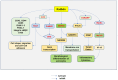
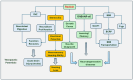
Results: Radixin research has focused on its cell functions, activation, and pathogenic roles in some diseases. Radixin and other ERM proteins maintain cell shape, growth, and motility. In the nervous system, radixin has been shown to prevent neurodegeneration and axonal growth. The activation of radixin is through phosphorylation of its conserved threonine residues. Radixin functions in cell signaling pathways by binding to membrane proteins and relaying the cell signals into the cells. Deficiency of radixin has been involved in the pathogenic process of diseases in the central nervous system and diabetic peripheral nerve injury. Moreover, radixin also plays a role in cell growth and drug resistance in multiple cancers. The trials of therapeutic potential through radixin modulation have been accumulating. However, the exact mechanisms underlying the roles of radixin are far from clarification.
Conclusions: Radixin plays various roles in cells and is involved in developing neurodegenerative diseases and many types of cancers. Therefore, radixin may be considered a potential target for developing therapeutic strategies for its related diseases. Further elucidation of the function and the cell signaling pathways that are linked to radixin may open the avenue to finding novel therapeutic strategies for diseases in the nervous system and other body systems.
Keywords: ERM; cancer; neurodegeneration; peripheral nerve injury; phosphorylation; radixin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

