Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia
- PMID: 39457654
- PMCID: PMC11504003
- DOI: 10.3390/biomedicines12102343
Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia
Abstract
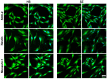
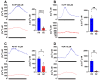
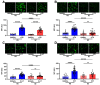
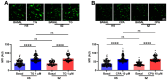
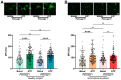
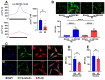
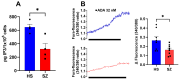
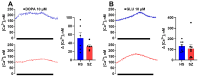
Background: Schizophrenia (SZ) is a multifactorial chronic psychiatric disorder with a worldwide prevalence of 1%. Altered expression of PLCβ occurs in SZ patients, suggesting alterations in the PLCβ/IP3/Ca2+ signaling pathway. This cascade regulates critical cellular processes in all cell types, including the neuronal lineage; however, there is scarce evidence regarding the functionality of this transduction signaling in neuronal cells derived from SZ patients. Objective: We evaluated the functionality of the PLCβ/IP3/Ca2+ pathway in olfactory neuronal precursor cells (hONPCs) obtained from SZ patients. Methods: Cryopreserved hONPCs isolated from SZ patients and healthy subjects (HS) were thawed. The cellular types in subcultures were corroborated by immunodetection of the multipotency and lineage markers SOX-2, Musashi-1, nestin, and β-III tubulin. The PLCβ/IP3/Ca2+ pathway was activated by GPCR (Gq) ligands (ATP, UTP, serotonin, and epinephrine). In addition, PLCβ and IP3R were directly stimulated by perfusing cells with the activators m-3M3FBS and ADA, respectively. Cytosolic Ca2+ was measured by microfluorometry and by Ca2+ imaging. The amount and subcellular distribution of the PLCβ1 and PLCβ3 isoforms were evaluated by confocal immunofluorescence. IP3 concentration was measured by ELISA. Results: The results show that the increase of cytosolic Ca2+ triggered by GPCR ligands or directly through either PLCβ or IP3R activation was significantly lower in SZ-derived hONPCs, regarding HS-derived cells. Moreover, the relative amount of the PLCβ1 and PLCβ3 isoforms and IP3 production stimulated with m-3M3FBS were reduced in SZ-derived cells. Conclusions: Our results suggest an overall functional impairment in the PLCβ/IP3/Ca2+ signaling pathway in SZ-derived hONPCs.
Keywords: IP3; PLCβ; calcium signaling; human olfactory neuronal stem cells; schizophrenia.
Conflict of interest statement
The authors have declared that there are no conflicts of interest concerning the subject of this study.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

