The Prospect of Improving Pancreatic Cancer Diagnostic Capabilities by Implementing Blood Biomarkers: A Study of Evaluating Properties of a Single IL-8 and in Conjunction with CA19-9, CEA, and CEACAM6
- PMID: 39457656
- PMCID: PMC11505492
- DOI: 10.3390/biomedicines12102344
The Prospect of Improving Pancreatic Cancer Diagnostic Capabilities by Implementing Blood Biomarkers: A Study of Evaluating Properties of a Single IL-8 and in Conjunction with CA19-9, CEA, and CEACAM6
Abstract
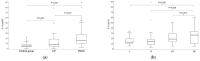
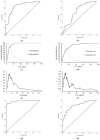
Background/Objectives: This study aims to evaluate the possible clinical application of interleukin 8 (IL-8) as a single biomarker and its capabilities in combination with carbohydrate antigen (CA19-9), carcinoembryonic antigen (CEA), and carcinoembryonic antigen cell adhesion molecule 6 (CEACAM6) as diagnostic and prognostic tools for pancreatic ductal adenocarcinoma (PDAC). Methods: A total of 170 serum samples from patients with PDAC (n = 100), chronic pancreatitis (CP) (n = 39), and healthy individuals (n = 31) were analysed. IL-8 and CEACAM6 were measured by an enzyme-linked immunosorbent assay (ELISA). CA19-9 and CEA were determined by chemiluminescent microparticle immunoassay, and bilirubin was quantified using a diazonium salt reaction. Receiver operating characteristic curve analysis, logistic regression, and Kaplan-Meier analyses were performed to evaluate the properties of a single IL-8 and in combination with other biomarkers. Results: The concentrations of IL-8 were statistically significantly higher in the PDAC group compared to the CP and control groups. Heterogeneous levels of IL-8 correlated with PDAC stages (p = 0.007). IL-8 had good and satisfactory diagnostic efficacy in differentiating PDAC from controls (0.858; p < 0.001) and patients with CP (0.696; p < 0.001), respectively. High and low expressions of IL-8 were not significantly associated with overall survival (OS) or disease-free survival (DFS). A combination of IL-8, CEACAM6, and CA19-9 reached the highest AUC values for differentiating PDAC from the control group. The best classification score between PDAC and the control group with CP patients was obtained by merging IL-8 and CA19-9 (0.894; p < 0.001). Conclusions: These results provide compelling evidence of IL-8 as a promising diagnostic biomarker. Nonetheless, due to the high complexity of PDAC, only the conjunction of IL-8, CA19-9, and CEACAM6 integrates sufficient diagnostic capabilities.
Keywords: IL-8; chronic pancreatitis; diagnostic biomarker; obstructive jaundice; pancreatic cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- ECIS Cancer Burden Statistics and Trends across Europe. [(accessed on 17 July 2024)]. Available online: https://ecis.jrc.ec.europa.eu/
LinkOut - more resources
Full Text Sources
Miscellaneous

