Intestinal Region-Dependent Impact of NFκB-Nrf Crosstalk in Myenteric Neurons and Adjacent Muscle Cells in Type 1 Diabetic Rats
- PMID: 39457659
- PMCID: PMC11504535
- DOI: 10.3390/biomedicines12102347
Intestinal Region-Dependent Impact of NFκB-Nrf Crosstalk in Myenteric Neurons and Adjacent Muscle Cells in Type 1 Diabetic Rats
Abstract
Background/objectives: Type 1 diabetes affects cytokines as potential inducers of NFκB signalling involved in inflammation and neuronal survival. Our goal was to assess the expression of NFκB p65 and its negative regulator, Nrf2, in myenteric neurons and adjacent smooth muscle of different gut segments after chronic hyperglycaemia and immediate insulin treatment.
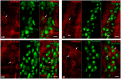
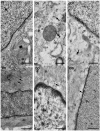
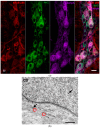
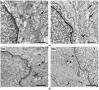
Methods: After ten weeks of hyperglycaemia, intestinal samples of control, streptozotocin-induced diabetic and insulin-treated diabetic rats were prepared for fluorescent immunohistochemistry, immunogold electron microscopy, ELISA and qPCR.
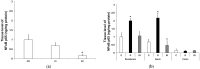
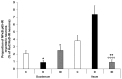
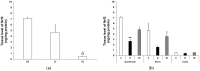
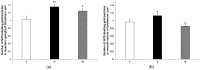
Results: In the diabetic rats, the proportion of NFκB p65-immunoreactive myenteric neurons decreased significantly in the duodenum and increased in the ileum. The density of NFκB p65-labelling gold particles increased in the ileal but remained unchanged in the duodenal ganglia. Meanwhile, both total and nuclear Nrf2 density increased in the myenteric neurons of the diabetic duodenum. In smooth muscle, NFκB p65 and Nrf2 density increased in the small intestine of diabetic rats. While on the mRNA level, NFκB p65 and Nrf2 were induced, on the protein level, NFκB p65 increased and Nrf2 decreased in muscle/myenteric plexus homogenates. Insulin treatment had protective effects.
Conclusions: Our findings reveal a segment-specific NFκB and Nrf expression in myenteric neurons and ganglionic muscular environments, which may contribute to regional neuronal survival and motility disturbances in diabetes.
Keywords: NFκB; Nrf2; animal model; enteric neurons; gut segments; hyperglycaemia; insulin; intestinal smooth muscle; myenteric plexus; type 1 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Diabetes. [(accessed on 16 September 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
-
- Modarelli R., Brown L., Boyd J., Podd B., Willis Z., Levenson A. Severe Multiple Organ Failure as a Consequence of Diabetic Ketoacidosis in an Adolescent with New-Onset Type 1 Diabetes: A Case Report. SAGE Open Med. Case Rep. 2023;11:2050313X231190004. doi: 10.1177/2050313X231190004. - DOI - PMC - PubMed
-
- Avagimyan A., Fogacci F., Pogosova N., Kakrurskiy L., Kogan E., Urazova O., Kobalava Z., Mikhaleva L., Vandysheva R., Zarina G., et al. Diabetic Cardiomyopathy: 2023 Update by the International Multidisciplinary Board of Experts. Curr. Probl. Cardiol. 2024;49:102052. doi: 10.1016/j.cpcardiol.2023.102052. - DOI - PubMed
Grants and funding
- No. FK131789 (N.B.)/Hungarian NKFIH fund project
- János Bolyai Research Scholarship (N.B.)/Hungarian Academy of Sciences
- ÚNKP-22-3-SZTE-389 (B.P.B.)/New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund
- ÚNKP-23-3-SZTE-426 (B.P.B.)/New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund
LinkOut - more resources
Full Text Sources

