The Effectiveness of NP001 on the Long-Term Survival of Patients with Amyotrophic Lateral Sclerosis
- PMID: 39457678
- PMCID: PMC11504292
- DOI: 10.3390/biomedicines12102367
The Effectiveness of NP001 on the Long-Term Survival of Patients with Amyotrophic Lateral Sclerosis
Abstract
Background/objectives: The aim of this study was to estimate the effect of a 6 months' treatment course of the innate immune modulator NP001 (a pH-adjusted intravenous formulation of purified sodium chlorite), on disease progression, as measured by overall survival (OS) in patients with amyotrophic lateral sclerosis.
Methods: Blinded survival data were retrospectively collected for 268 of the 273 patients who had participated in two phase 2 placebo-controlled clinical trials of NP001 (ClinicalTrials.gov: NCT01281631 and NCT02794857) and received at least one dose of either 1 mg/kg or 2 mg/kg of NP001 as chlorite based on actual body weight, or placebo. Kaplan-Meier methods were used on the intent-to-treat population to estimate survival probabilities.
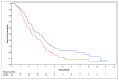
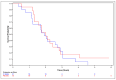
Results: In the overall population, the median OS was 4.8 months (2.7 years [95% CI: 2.3, 3.5] in the 2 mg/kg NP001group and 2.3 years [95% CI: 1.8, 2.9] in the placebo group). Hazard ratio (HR): 0.77 (95% CI: 0.57, 1.03), p = 0.073. Among patients aged ≤ 65 years, the median OS for the 2 mg/kg NP001 group was 10.8 months (3.3 years [95% CI: 2.4, 3.8] in the 2 mg/kg NP001 group and 2.4 years [95% CI: 1.7, 3.3] in the placebo group). HR: 0.69 (95% CI: 0.50, 0.95). No differences were observed in the 1 mg/kg NP001 group or in patients aged > 65 years.
Conclusions: The findings from this study suggest that a 6 months' treatment course of NP001 resulted in a 4.8-month increase in overall survival in patients with ALS. The findings from this study indicate that targeting inflammation associated with the innate immune system may provide a pathway for new therapeutic options for the treatment of ALS.
Keywords: amyotrophic lateral sclerosis; innate immunity; neuroinflammation; overall survival; sodium chlorite.
Conflict of interest statement
B.D.F. is an employee of Hudson Innovations, LLC, which received consultation fees, stock and stock options from Neuvivo, Inc. as compensation for services provided. Hudson Innovations, LLC provides consultation services in pharmaceutical development across the industry, for which it is compensated. B.D.F. has also been listed as an inventor on patent applications filed by Neuvivo, Inc. N.A.G. is a study investigator and has received grants from Abcuro, Alexion, Amylyx, Anelixis, Annexon, Calico, Clene, Cytokinetics, Fulcrum, Healey, Janssen, Kezar, Medicinova, MT Pharma, PTC, Roche, Sanofi, and Transposon; has served on Advisory Boards for Abcuro, Alexion, Amylyx, Annexon, Argenx, Astrazeneca, CSL Behring, Fulcrum, Kezar, MT Pharma, Sanofi Genzyme, and UCB; has received travel reimbursement and honoraria; and has served on the speaker’s bureau for Argenx and CSL. T.R.F. is a Professor of Biostatistics at the University of Washington; received consultation fees from Neuvivo, Inc; is a consultant to government and industry sponsors in the design, conduct and analysis of clinical trials; and had full access to all data. P.M.B. is an employee of the University of California, San Francisco; and has received stock options as compensation for services provided. N.R.B. and Z.K. are employees of Pharmaceutical Product Development, LLC, Part of Thermo Fisher Scientific, which was engaged by Neuvivo, Inc., for which it was compensated; and had full access to all data. M.R. is an employee of Engage Management Solutions, LLC, which received consultation fees from Neuvivo, Inc. Engage Management Solutions, LLC provides consultation services in pharmaceutical development across the industry, for which it is compensated. A.A. is a founder of Neuvivo, Inc. and receives a salary, stock and stock options in Neuvivo, Inc.; and has also been listed as an inventor on patent applications filed by Neuvivo, Inc. M.M is a founder of Neuvivo, Inc. and receives a salary, stock and stock options in Neuvivo, Inc.; and has also been listed as an inventor on patent applications filed by Neuvivo, Inc. The funders participated in the study design, making the original trial data sources accessible to the investigators as described, funded the data collection and analyses, reviewed the manuscript, and participated in but were not the sole contributors in the decision to submit this manuscript.
Figures

References
-
- Kjældgaard A.L., Pilely K., Olsen K.S., Jessen A.H., Lauritsen A.Ø., Pedersen S.W., Svenstrup K., Karlsborg M., Thagesen H., Blaabjerg M., et al. Prediction of survival in amyotrophic lateral sclerosis: A nationwide, Danish cohort study. BMC Neurol. 2021;21:164. doi: 10.1186/s12883-021-02187-8. - DOI - PMC - PubMed
-
- Miller R.G., Zhang R., Block G., Katz J., Barohn R., Kasarskis E., Forshew D., Gopalakrishnan V., McGrath M.S. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph. Lateral Scler. Front. Degener. 2014;15:601–609. doi: 10.3109/21678421.2014.951940. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

