Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart-Brain Axis
- PMID: 39457698
- PMCID: PMC11504205
- DOI: 10.3390/biomedicines12102387
Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart-Brain Axis
Abstract
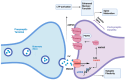
Within the central nervous system, synaptic plasticity, fundamental to processes like learning and memory, is largely driven by activity-dependent changes in synaptic strength. This plasticity often manifests as long-term potentiation (LTP) and long-term depression (LTD), which are bidirectional modulations of synaptic efficacy. Strong epidemiological and experimental evidence show that the heart-brain axis could be severely compromised by both neurological and cardiovascular disorders. Particularly, cardiovascular disorders, such as heart failure, hypertension, obesity, diabetes and insulin resistance, and arrhythmias, may lead to cognitive impairment, a condition known as cardiogenic dementia. Herein, we review the available knowledge on the synaptic and molecular mechanisms by which cardiogenic dementia may arise and describe how LTP and/or LTD induction and maintenance may be compromised in the CA1 region of the hippocampus by heart failure, metabolic syndrome, and arrhythmias. We also discuss the emerging evidence that endothelial dysfunction may contribute to directly altering hippocampal LTP by impairing the synaptically induced activation of the endothelial nitric oxide synthase. A better understanding of how CV disorders impact on the proper function of central synapses will shed novel light on the molecular underpinnings of cardiogenic dementia, thereby providing a new perspective for more specific pharmacological treatments.
Keywords: NMDA receptors; arrhythmias; cardiogenic dementia; cardiovascular disorders; cognitive impairment; heart failure; heart–brain axis; long-term potentiation; metabolic syndrome; synaptic plasticity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

