Absolute Quantitative Targeted Monitoring of Potential Plasma Protein Biomarkers: A Pilot Study on Healthy Individuals
- PMID: 39457715
- PMCID: PMC11504031
- DOI: 10.3390/biomedicines12102403
Absolute Quantitative Targeted Monitoring of Potential Plasma Protein Biomarkers: A Pilot Study on Healthy Individuals
Abstract
Background/objectives: The development of blood tests for the early detection of individual predisposition to socially significant diseases remains a pressing issue.
Methods: In this pilot study, multiple reaction monitoring mass spectrometry (MRM-MS) with a BAK-270 assay was applied for protein concentrations analysis in blood plasma from 21 healthy volunteers of the European cohort.
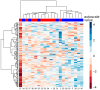
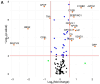
Results: The levels of 138 plasma proteins were reliably and precisely quantified in no less than 50% of samples. The quantified proteins included 66 FDA-approved markers of cardiovascular diseases (CVD), and other potential biomarkers of pathologies such as cancer, diabetes mellitus, and Alzheimer's disease. The analysis of individual variations of the plasma proteins revealed significant differences between the male (11) and female (10) groups. In total, fifteen proteins had a significantly different concentration in plasma; this included four proteins that exhibited changes greater than ±1.5-fold, three proteins (RBP4, APCS, and TTR) with higher levels in males, and one (SHBG) elevated in females. The obtained results demonstrated considerable agreement with the data collected from 20 samples of a North American cohort, which were analyzed with the similar MRM assay. The most significant differences between the cohorts of the two continents were observed in the level of 42 plasma proteins (including 24 FDA markers), of which 17 proteins showed a ≥1.5-fold change, and included proteins increased in North Americans (APOB, CRTAC1, C1QB, C1QC, C9, CRP, HP, IGHG1, IGKV4-1, SERPING1, RBP4, and AZGP1), as well as those elevated in Europeans (APOF, CD5L, HBG2, SELPLG, and TNA).
Conclusions: The results suggest a different contribution of specific (patho)physiological pathways (e.g., immune system and blood coagulation) to the development of socially significant diseases in Europeans and North Americans, and they should be taken into account when refining diagnostic panels.
Keywords: MRM; biomarkers; blood; healthy volunteers; mass spectrometry; plasma; proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ngo D., Sinha S., Shen D., Kuhn E.W., Keyes M.J., Shi X., Benson M.D., O’Sullivan J.F., Keshishian H., Farrell L.A., et al. Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation. 2016;134:270–285. doi: 10.1161/CIRCULATIONAHA.116.021803. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

