Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows
- PMID: 39457858
- PMCID: PMC11503749
- DOI: 10.3390/ani14202929
Mitochondria-Targeted Antioxidant MitoQ Improves In Vitro Maturation and Subsequent Embryonic Development from Culled Cows
Abstract
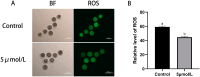
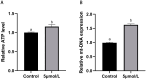
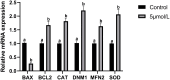
The purpose of this study was to investigate the effects and mechanisms of MitoQ on the IVM of culled bovine oocytes and subsequent embryonic development. The results revealed that in comparison to the control group (0 µmol/L), the IVM rate (p < 0.05) and subsequent blastocyst rate (p < 0.05) of the low-concentration 1 and 5 µmol/L MitoQ treatment group were increased. The level of ROS (p < 0.05) in the MitoQ treatment group was decreased in comparison to the control group. Additionally, the level of GSH, MMP, ATP, and mt-DNA in the MitoQ treatment group was increased (p < 0.05) in comparison to the control group. The expression level of BAX was decreased (p < 0.05) in the MitoQ treatment group, and the BCL2, DNM1, Mfn2, SOD, and CAT were increased (p < 0.05). In conclusion, MitoQ improved mitochondrial dysfunction, increased mitochondrial activity during IVM, and reduced oxidative stress, resulting in increased IVM rates and subsequent embryonic development from culled cows.
Keywords: IVM; MitoQ; antioxidant; bovine oocytes; mitochondria; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Risks of using mitoquinone during in vitro maturation and its potential protective effects against lipotoxicity-induced oocyte mitochondrial stress.J Assist Reprod Genet. 2024 Feb;41(2):371-383. doi: 10.1007/s10815-023-02994-7. Epub 2023 Dec 26. J Assist Reprod Genet. 2024. PMID: 38146030 Free PMC article.
-
Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes.Hum Reprod. 2021 Feb 18;36(3):771-784. doi: 10.1093/humrep/deaa300. Hum Reprod. 2021. PMID: 33367783 Free PMC article.
-
Exposing Mouse Oocytes to MitoQ During In Vitro Maturation Improves Maturation and Developmental Competence.Iran J Biotechnol. 2020 Jul 1;18(3):e2454. doi: 10.30498/IJB.2020.154641.2454. eCollection 2020 Jul. Iran J Biotechnol. 2020. PMID: 33850943 Free PMC article.
-
Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model.Hum Reprod. 2019 Oct 2;34(10):1984-1998. doi: 10.1093/humrep/dez161. Hum Reprod. 2019. PMID: 31625574
-
Effects of limonin on oxidative stress and early apoptosis in oocytes during in vitro maturation.Theriogenology. 2024 Apr 1;218:8-15. doi: 10.1016/j.theriogenology.2024.01.025. Epub 2024 Jan 24. Theriogenology. 2024. PMID: 38290232
Cited by
-
Can the Supplementation of Oocytes with Extra Copies of mtDNA Impact Development Without Being Transmitted? A Molecular Account.Int J Mol Sci. 2025 Mar 18;26(6):2746. doi: 10.3390/ijms26062746. Int J Mol Sci. 2025. PMID: 40141388 Free PMC article.
References
-
- Amoushahi M., Salehnia M., Ghorbanmehr N. The mitochondrial DNA copy number, cytochrome c oxidase activity and reactive oxygen species level in metaphase II oocytes obtained from in vitro culture of cryopreserved ovarian tissue in comparison with in vivo-obtained oocyte. J. Obstet. Gynaecol. Res. 2018;44:1937–1946. doi: 10.1111/jog.13747. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

