Temporal Changes in Jejunal and Ileal Microbiota of Broiler Chickens with Clinical Coccidiosis (Eimeria maxima)
- PMID: 39457906
- PMCID: PMC11503835
- DOI: 10.3390/ani14202976
Temporal Changes in Jejunal and Ileal Microbiota of Broiler Chickens with Clinical Coccidiosis (Eimeria maxima)
Abstract
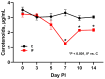
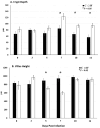
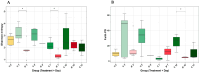
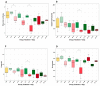
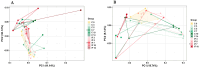
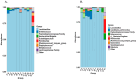
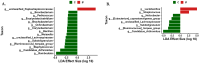
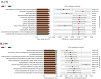
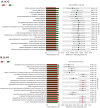
Coccidiosis in broiler chickens continues to be a major disease of the gastrointestinal tract, causing economic losses to the poultry industry worldwide. The goal of this study was to generate a symptomatic Eimeria maxima (1000 oocysts) infection to determine its effect on the luminal and mucosal microbiota populations (L and M) in the jejunum and ileum (J and IL). Samples were taken from day 0 to 14 post-infection, and sequencing of 16S rRNA was performed using Illumina technology. Infected birds had significantly (p < 0.0001) lower body weight gain (BWG), higher feed conversion ratio (FCR) (p = 0.0015), increased crypt depth, and decreased villus height (p < 0.05). The significant differences in alpha and beta diversity were observed primarily at height of infection (D7). Analysis of taxonomy indicated that J-L and M were dominated by Lactobacillus, and in IL-M, changeover from Candidatus Arthromitus to Lactobacillus as the major taxon was observed, which occurred quicky in infected animals. LEfSe analysis found that in the J-M of infected chickens, Lactobacillus was significantly more abundant in infected (IF) chickens. These findings show that E. maxima infection affects the microbiota of the small intestine in a time-dependent manner, with different effects on the luminal and mucosal populations.
Keywords: 16S rRNA sequencing; Eimeria maxima; broilers; coccidiosis; gut morphology; microbiota.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Blake D.P., Vrba V., Xia D., Jatau I.D., Spiro S., Nolan M.J., Underwood G., Tomley F.M. Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus) Int. J. Parasitol. 2021;51:621–634. doi: 10.1016/j.ijpara.2020.12.004. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

