Analysis of Differential Alternative Splicing in Largemouth Bass After High Temperature Exposure
- PMID: 39457935
- PMCID: PMC11505094
- DOI: 10.3390/ani14203005
Analysis of Differential Alternative Splicing in Largemouth Bass After High Temperature Exposure
Abstract
Temperature is one of the critical factors affecting the physiological functions of fish. With ongoing global warming, changes in water temperature have a profound impact on fish species. Alternative splicing, being a significant mechanism for gene expression regulation, facilitates fish to adapt and thrive in dynamic and varied aquatic environments. Our study used transcriptome sequencing to analyze alternative splicing in largemouth bass gills at 34 °C for 24 h. The findings indicated an increase in both alternative splicing events and alternative splicing genes after high temperature treatment. Specifically, the comparative analysis revealed a total of 674 differential alternative splicing events and 517 differential alternative splicing genes. Enrichment analysis of differential alternative splicing genes revealed significant associations with various gene ontology (GO) terms and KEGG pathways, particularly in immune-related pathways like necroptosis, apoptosis, and the C-type lectin receptor signaling pathway. These results emphasize that some RNA splicing-related genes are involved in the response of largemouth bass to high temperatures.
Keywords: differential alternative splicing; environmental stress; largemouth bass; transcriptome sequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
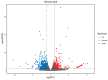





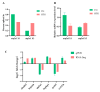
References
-
- Stilwell N.K., Frasca S.J., Farina L.L., Subramaniam K., Imnoi K., Viadanna P.H., Hopper L., Powell J., Colee J., Waltzek T.B. Effect of water temperature on frog virus 3 disease in hatchery-reared pallid sturgeon Scaphirhynchus albus. Dis. Aquat. Organ. 2022;148:73–86. doi: 10.3354/dao03645. - DOI - PubMed
-
- Huang J., Zhang X., Zhang Q., Lin Y., Hao M., Luo Y., Zhao Z., Yao Y., Chen X., Wang L., et al. Recently amplified arctic warming has contributed to a continual global warming trend. Nat. Clim. Chang. 2017;7:875–879. doi: 10.1038/s41558-017-0009-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

