Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population
- PMID: 39457994
- PMCID: PMC11509035
- DOI: 10.3390/jcm13206044
Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population
Abstract
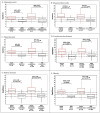
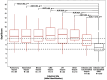
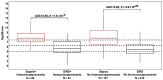
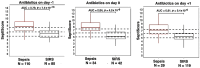
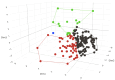
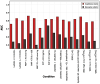
Background/Objective: SeptiCyte RAPID is a transcriptional host response assay that discriminates between sepsis and non-infectious systemic inflammation (SIRS) with a one-hour turnaround time. The overall performance of this test in a cohort of 419 patients has recently been described [Balk et al., J Clin Med 2024, 13, 1194]. In this study, we present the results from a detailed stratification analysis in which SeptiCyte RAPID performance was evaluated in the same cohort across patient groups and subgroups encompassing different demographics, comorbidities and disease, sources and types of pathogens, interventional treatments, and clinically defined phenotypes. The aims were to identify variables that might affect the ability of SeptiCyte RAPID to discriminate between sepsis and SIRS and to determine if any patient subgroups appeared to present a diagnostic challenge for the test. Methods: (1) Subgroup analysis, with subgroups defined by individual demographic or clinical variables, using conventional statistical comparison tests. (2) Principal component analysis and k-means clustering analysis to investigate phenotypic subgroups defined by unique combinations of demographic and clinical variables. Results: No significant differences in SeptiCyte RAPID performance were observed between most groups and subgroups. One notable exception involved an enhanced SeptiCyte RAPID performance for a phenotypic subgroup defined by a combination of clinical variables suggesting a septic shock response. Conclusions: We conclude that for this patient cohort, SeptiCyte RAPID performance was largely unaffected by key variables associated with heterogeneity in patients suspected of sepsis.
Keywords: SIRS; SeptiCyte; host immune response; phenotype; sepsis; sepsis likelihood; stratification.
Conflict of interest statement
K.N., T.D.Y., D.S., J.T.K., S.C., R.F.D. and R.B.B. declare they are present or past employees or shareholders of Immunexpress, Inc. X.W.M. declares that she is a present employee of Princeton Pharmatech, Inc. R.R.M. III discloses that, over the time period of relevance to this work he was paid (as a consultant) an honorarium by Immunexpress for conducting a critical review of a Clinical Evaluation Report of SeptiCyte technology, submitted by Immunexpress to the Therapeutic Goods Administration (TGA) of Australia. N.R.A. declares that he has received grants from the NIH and Department of Defense for non-related work and that payments from these grants are made to his institution, the University of Colorado. N.R.A. also declares that he is a committee member of the Pfizer Paxlovid U.S. Medical Advisory Committee formed to broadly discuss COVID-19-related therapeutic priorities and populations of interest. B.K.L. declares that he is a consultant for Karius Inc., is a member of the Scientific Advisory Board for Seegene Inc., and has received honoraria for speaking for Qiagen Inc. No other competing interests are declared.
Figures

References
-
- Liu V.X., Bhimarao M., Greene J.D.M., Manickam R.N., Martinez A., Schuler A., Barreda F.M., Escobar G.J. The Presentation, Pace, and Profile of Infection and Sepsis Patients Hospitalized Through the Emergency Department: An Exploratory Analysis. Crit. Care Explor. 2021;3:e0344. doi: 10.1097/CCE.0000000000000344. - DOI - PMC - PubMed
-
- Seymour C.W., Kennedy J.N., Wang S., Chang C.-C.H., Elliott C.F., Xu Z., Berry S., Clermont G., Cooper G., Gomez H., et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

