Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series
- PMID: 39458022
- PMCID: PMC11509003
- DOI: 10.3390/jcm13206072
Intravenous Infusion of Autologous Mesenchymal Stem Cells Expanded in Auto Serum for Chronic Spinal Cord Injury Patients: A Case Series
Abstract
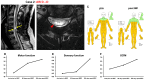
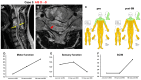
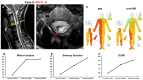
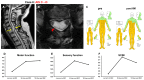
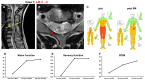
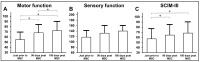
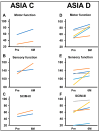
Objective: The safety, feasibility, and potential functional improvement following the intravenous infusion of mesenchymal stem cells (MSCs) were investigated in patients with chronic severe spinal cord injury (SCI). Methods: The intravenous infusion of autologous MSCs cultured in auto-serum under Good Manufacturing Practices (GMP) was administered to seven patients with chronic SCI (ranging from 1.3 years to 27 years after the onset of SCI). In addition to evaluating feasibility and safety, neurological function was evaluated using the American Spinal Injury Association Impairment Scale (AIS), International Standards for Neurological Classification of Spinal Cord Injury (ISCSCI-92), and Spinal Cord Independence Measure III (SCIM-III). Results: No serious adverse events occurred. Neither CNS tumors, abnormal cell growth, nor neurological deterioration occurred in any patients. While this initial case series was not blinded, significant functional improvements and increased quality of life (QOL) were observed at 90 and 180 days post-MSC infusion compared to pre-infusion status. One patient who had an AIS grade C improved to grade D within six months after MSC infusion. Conclusions: This case series suggests that the intravenous infusion of autologous MSCs is a safe and feasible therapeutic approach for chronic SCI patients. Furthermore, our data showed significant functional improvements and better QOL after MSC infusion in patients with chronic SCI. A blind large-scale study will be necessary to fully evaluate this possibility.
Keywords: case series; chronic; clinical trials; intravenous; mesenchymal stem cell; spinal cord injury.
Conflict of interest statement
The Department of Advanced Regenerative Therapeutics at Sapporo Medical University has been endowed by the NIPRO Corporation since 1st February 2014. Sapporo Medical University and NIPRO Corporation have entered into a joint research and development agreement since 1st April 2014, which provides research support to the Department, including work carried out by some of the co-authors (M. Sasaki, M. Nakazaki, Y. Kataoka-Sasaki, S. Oka and O. Honmou). R. Hirota, T. Morita, M. Nakazaki and J. D. Kocsis received research support through Yale University from NIPRO Corporation on pre-clinical studies of rodent MSCs in animal models. R. Hirota, M. Sasaki, S. Oka, R. Fukushi, T. Ogata, A. Teramoto, T. Yamashita and O. Honmou received honoraria from NIPRO Corporation. S. Iyama, K. Kurihara, H. Obara, T. Oshigiri, T. Namioka, A. Namioka, R. Onodera, M. Takemura, R. Ukai, T. Yokoyama, Y. Sasaki, Tatsuro Yamashita, M. Kobayashi, Y. Okuma, R. Kondo, R. Aichi, S. Ohmatsu, N. Kawashima, Y. M. Ito, M. Kobune, K. Takada, S. Ishiai report no competing interests on this study.
Figures



References
-
- James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., Abbasi N., Abdulkader R., Abraha H.N., Adsuar J.C., et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. - DOI - PMC - PubMed
-
- Levi A.D., Anderson K.D., Okonkwo D.O., Park P., Bryce T.N., Kurpad S.N., Aarabi B., Hsieh J., Gant K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma. 2019;36:891–902. doi: 10.1089/neu.2018.5843. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

