The Gut Microbiome in Sepsis: From Dysbiosis to Personalized Therapy
- PMID: 39458032
- PMCID: PMC11508704
- DOI: 10.3390/jcm13206082
The Gut Microbiome in Sepsis: From Dysbiosis to Personalized Therapy
Abstract
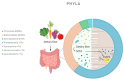
Sepsis is a complex clinical syndrome characterized by an uncontrolled inflammatory response to an infection that may result in septic shock and death. Recent research has revealed a crucial link between sepsis and alterations in the gut microbiota, showing that the microbiome could serve an essential function in its pathogenesis and prognosis. In sepsis, the gut microbiota undergoes significant dysbiosis, transitioning from a beneficial commensal flora to a predominance of pathobionts. This transformation can lead to a dysfunction of the intestinal barrier, compromising the host's immune response, which contributes to the severity of the disease. The gut microbiota is an intricate system of protozoa, fungi, bacteria, and viruses that are essential for maintaining immunity and metabolic balance. In sepsis, there is a reduction in microbial heterogeneity and a predominance of pathogenic bacteria, such as proteobacteria, which can exacerbate inflammation and negatively influence clinical outcomes. Microbial compounds, such as short-chain fatty acids (SCFAs), perform a crucial task in modulating the inflammatory response and maintaining intestinal barrier function. However, the role of other microbiota components, such as viruses and fungi, in sepsis remains unclear. Innovative therapeutic strategies aim to modulate the gut microbiota to improve the management of sepsis. These include selective digestive decontamination (SDD), probiotics, prebiotics, synbiotics, postbiotics, and fecal microbiota transplantation (FMT), all of which have shown potential, although variable, results. The future of sepsis management could benefit greatly from personalized treatment based on the microbiota. Rapid and easy-to-implement tests to assess microbiome profiles and metabolites associated with sepsis could revolutionize the disease's diagnosis and management. These approaches could not only improve patient prognosis but also reduce dependence on antibiotic therapies and promote more targeted and sustainable treatment strategies. Nevertheless, there is still limited clarity regarding the ideal composition of the microbiota, which should be further characterized in the near future. Similarly, the benefits of therapeutic approaches should be validated through additional studies.
Keywords: DAO (diamine oxidase); FMT (fecal microbiota transplantation); I-FABP (intestinal fatty acid-binding protein); SDD (selective digestive decontamination); antibiotics; intestinal barrier; intestinal microbiota; postbiotics; prebiotics; probiotics; sepsis; symbiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Postbiotics as potential new therapeutic agents for sepsis.Burns Trauma. 2023 Jun 15;11:tkad022. doi: 10.1093/burnst/tkad022. eCollection 2023. Burns Trauma. 2023. PMID: 37334140 Free PMC article. Review.
-
Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances.Front Immunol. 2023 Jan 11;13:1063543. doi: 10.3389/fimmu.2022.1063543. eCollection 2022. Front Immunol. 2023. PMID: 36713461 Free PMC article.
-
Gut-targeted therapies for type 2 diabetes mellitus: A review.World J Clin Cases. 2024 Jan 6;12(1):1-8. doi: 10.12998/wjcc.v12.i1.1. World J Clin Cases. 2024. PMID: 38292634 Free PMC article. Review.
-
Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics.Nutrients. 2024 Nov 19;16(22):3955. doi: 10.3390/nu16223955. Nutrients. 2024. PMID: 39599742 Free PMC article. Review.
-
A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity.J Clin Med. 2025 May 23;14(11):3673. doi: 10.3390/jcm14113673. J Clin Med. 2025. PMID: 40507435 Free PMC article. Review.
Cited by
-
Unveiling the Therapeutic Potential of Probiotics in Sepsis: A Review.Food Sci Nutr. 2025 Jun 8;13(6):e70364. doi: 10.1002/fsn3.70364. eCollection 2025 Jun. Food Sci Nutr. 2025. PMID: 40491980 Free PMC article. Review.
-
Desulfovibrio vulgaris exacerbates sepsis by inducing inflammation and oxidative stress in multiple organs.Front Microbiol. 2025 Apr 30;16:1574998. doi: 10.3389/fmicb.2025.1574998. eCollection 2025. Front Microbiol. 2025. PMID: 40371102 Free PMC article.
-
Analysis of ICU resistome dynamics in patients, staff and environment for the identification of predictive biomarkers of sepsis and early mortality.Sci Rep. 2025 Jul 11;15(1):25080. doi: 10.1038/s41598-025-10848-8. Sci Rep. 2025. PMID: 40646273 Free PMC article.
-
Gut microbiota: A novel target for sepsis treatment.Chin Med J (Engl). 2025 Jul 5;138(13):1513-1515. doi: 10.1097/CM9.0000000000003630. Epub 2025 May 26. Chin Med J (Engl). 2025. PMID: 40419445 Free PMC article. No abstract available.
-
The Need for Standardized Guidelines for the Use of Monocyte Distribution Width (MDW) in the Early Diagnosis of Sepsis.J Pers Med. 2024 Dec 27;15(1):5. doi: 10.3390/jpm15010005. J Pers Med. 2024. PMID: 39852198 Free PMC article. Review.
References
-
- De Siena M., Laterza L., Matteo M.V., Mignini I., Schepis T., Rizzatti G., Ianiro G., Rinninella E., Cintoni M., Gasbarrini A. Gut and Reproductive Tract Microbiota Adaptation during Pregnancy: New Insights for Pregnancy-Related Complications and Therapy. Microorganisms. 2021;9:473. doi: 10.3390/microorganisms9030473. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

