Comparison of Oral Procainamide and Mexiletine Treatment of Recurrent and Refractory Ventricular Tachyarrhythmias
- PMID: 39458049
- PMCID: PMC11508758
- DOI: 10.3390/jcm13206099
Comparison of Oral Procainamide and Mexiletine Treatment of Recurrent and Refractory Ventricular Tachyarrhythmias
Abstract
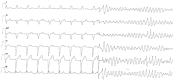
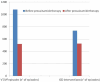
Background: Antiarrhythmic therapy for recurrent ventricular arrhythmias (VAs) in patients having undergone catheter ablation and in whom amiodarone and/or beta-blockers were ineffective or contraindicated is a controversial issue. Purpose: The present study sought to compare the efficacy and tolerability of oral procainamide and mexiletine in patients with recurrent ventricular arrhythmias when the standard therapy strategy failed. Methods: All patients with an implantable cardioverter defibrillator (ICD) treated with oral procainamide or mexiletine for recurrent ventricular tachycardia (VT) or ventricular fibrillation (VF) in two cardiology divisions between January 2010 and January 2020 were enrolled. Patients were divided into group A (oral procainamide) and group B (mexiletine) and the two groups were compared to each other. The primary endpoint was the efficacy of therapy; the secondary endpoint was the discontinuation of therapy. All events that occurred during procainamide or mexiletine treatment were compared with a matched duration period before the initiation of the therapy. Antiarrhythmic therapy was considered effective when a ≥80% reduction of the sustained ventricular arrhythmias burden recorded by the ICD was achieved. Results: A total of 68 consecutive patients (61 males, 89.7%; mean age 74 ± 10 years) were included in this retrospective analysis. After a median follow-up of 19 months, 38 (56%) patients had a significant reduction in the VA burden. After multivariable adjustment, therapy with procainamide was independently associated with an almost 3-fold higher efficacy on VA suppression compared to mexiletine (HR 2.54, 95% CI 1.06-6.14, p = 0.03). Only three patients (9%) treated with procainamide presented severe side effects (dyspnea or hypotension) requiring discontinuation of therapy compared with six patients (18%) treated with mexiletine who interrupted therapy because of severe side effects (p = 0.47). Conclusions: Compared to mexiletine, oral procainamide had a higher efficacy for the treatment of recurrent and refractory VAs, and showed a good profile of tolerability.
Keywords: antiarrhythmic therapies; arrhythmic burden; implantable cardioverter defibrillators; ventricular arrhythmias.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
-
- Moss A.J., Greenberg H., Case R.B., Zareba W., Hall W.J., Brown M.W., Daubert J.P., McNitt S., Andrews M.L., Elkin A.D. Multicenter Automatic Defibrillator Implantation. Trial-II (MADIT-II) Research Group. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. - DOI - PubMed
-
- Poole J.E., Johnson G.W., Hellcamp A.S., Anderson J., Callans D.J., Raitt M.H., Reddy R.K., Marchilinski F.E., Yee R., Guarnieri T., et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. - DOI - PMC - PubMed
-
- Sood N., Ruwald A.C., Solomon S., Daubert J.P., McNitt S., Polonsky B., Jons C., Clyne C.A., Zareba W., Moss A.J. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur. Heart J. 2014;35:106–115. doi: 10.1093/eurheartj/eht451. - DOI - PubMed
LinkOut - more resources
Full Text Sources

