Postoperative Outcomes of PreserFlo MicroShunt in Patients with Exfoliation Glaucoma
- PMID: 39458082
- PMCID: PMC11508753
- DOI: 10.3390/jcm13206132
Postoperative Outcomes of PreserFlo MicroShunt in Patients with Exfoliation Glaucoma
Abstract
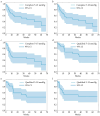
Objectives: This study aimed to evaluate the postoperative outcomes of the PreserFlo MicroShunt in Asian patients with exfoliation glaucoma. Methods: We used the Kaplan-Meier method to analyze 29 eyes of 29 patients with exfoliation glaucoma (mean age: 80.7 ± 8.3 years; 16 males; 24 eyes with intraocular lens implants; preoperative intraocular pressure [IOP]: 32.5 ± 9.3 mmHg; preoperative antiglaucoma medications: 3.4 ± 1.0; Asian ethnicity: 100%) who underwent PreserFlo MicroShunt surgery alone at Saneikai Tsukazaki Hospital from November 2022 to November 2023. The criteria for survival were a reduction in IOP of ≥20%, no additional glaucoma surgery, and IOP of 5-21 mmHg (condition 1), 5-18 mmHg (condition 2), and 5-15 mmHg (condition 3). Needling and glaucoma eye drops were considered qualified successes. Results: The mean follow-up period was 27.9 weeks, with a reoperation rate of 31% (9 cases). The complete and qualified success survival rates at 24 weeks were 56%, 52%, and 49%, and 67%, 59%, and 53% for conditions 1-3, respectively. The complete and qualified success survival rates at 48 weeks were 47%, 43%, and 45%, and 52%, 46%, and 48% for conditions 1-3, respectively. Conclusions: The postoperative outcomes of the PreserFlo MicroShunt in Asian patients with exfoliation glaucoma demonstrated an approximate 50% success rate at both 24 and 48 weeks, with a reoperation rate of approximately 30%. Caution is warranted when performing PreserFlo MicroShunt in patients with exfoliation glaucoma.
Keywords: exfoliation glaucoma; glaucoma surgery; innfocus; microshunt; open-angle glaucoma; preserflo; pseudoexfoliation glaucoma; subconjunctival filtration surgery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Effectiveness of MicroShunt in Patients with Primary Open-Angle and Pseudoexfoliative Glaucoma: A Retrospective European Multicenter Study.Ophthalmol Glaucoma. 2022 Mar-Apr;5(2):210-218. doi: 10.1016/j.ogla.2021.08.005. Epub 2021 Aug 31. Ophthalmol Glaucoma. 2022. PMID: 34478904
-
Clinical Outcomes and Cost Analysis of PreserFlo versus Trabeculectomy for Glaucoma Management in the United Kingdom.Ophthalmol Glaucoma. 2023 Jul-Aug;6(4):342-357. doi: 10.1016/j.ogla.2022.11.006. Epub 2022 Nov 23. Ophthalmol Glaucoma. 2023. PMID: 36427750
-
Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study.J Clin Med. 2022 Nov 29;11(23):7086. doi: 10.3390/jcm11237086. J Clin Med. 2022. PMID: 36498660 Free PMC article.
-
PreserFlo® MicroShunt: An Overview of This Minimally Invasive Device for Open-Angle Glaucoma.Vision (Basel). 2022 Feb 9;6(1):12. doi: 10.3390/vision6010012. Vision (Basel). 2022. PMID: 35225971 Free PMC article. Review.
-
Management practices and surgical techniques for ab externo less invasive glaucoma surgery: a literature review and expert recommendations.Graefes Arch Clin Exp Ophthalmol. 2025 May 8. doi: 10.1007/s00417-025-06843-4. Online ahead of print. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 40338299 Review.
Cited by
-
Short-term outcomes of the PreserFlo MicroShunt in Japanese patients with exfoliation glaucoma: a comparison with primary open-angle glaucoma using propensity score matching.Jpn J Ophthalmol. 2025 Aug 26. doi: 10.1007/s10384-025-01265-5. Online ahead of print. Jpn J Ophthalmol. 2025. PMID: 40856918
References
-
- Heijl A., Leske M.C., Bengtsson B., Hyman L., Bengtsson B., Hussein M., Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. - DOI - PubMed
LinkOut - more resources
Full Text Sources