Role of Liquid-Based Cytology in the Endoscopic Diagnosis of Pancreatic Ductal Adenocarcinoma
- PMID: 39458098
- PMCID: PMC11509073
- DOI: 10.3390/jcm13206148
Role of Liquid-Based Cytology in the Endoscopic Diagnosis of Pancreatic Ductal Adenocarcinoma
Abstract
Recently, endoscopic ultrasound-guided tissue acquisition (EUS-TA) has been widely used to diagnose pancreatic ductal adenocarcinoma (PDAC). The histological examination of core tissues acquired using novel biopsy needles is the primary diagnostic approach for patients with PDAC. However, in patients with early-stage PDAC, such as Stages 0 and I, EUS-TA can be challenging, and its diagnostic accuracy may be limited. This presents a clinical dilemma: The earlier that clinicians attempt to accurately diagnose PDAC, the more difficult it becomes to do so using EUS-TA. Liquid-based cytology (LBC) is a technique for preparing pathological specimens from liquefied cytology specimens by placing the collected material in a special fixative preservative fluid. LBC offers advantages, such as specimen optimization with reduced blood interference, a high cell-collection rate, and the simplicity of the procedure in the endoscopy room. The use of LBC may improve diagnostic accuracy, particularly for early-stage PDAC. Therefore, we emphasize that cytology remains a valuable tool for the endoscopic diagnosis of PDAC. In this review, we discuss the role of LBC in the endoscopic diagnosis of PDAC.
Keywords: cytology; endoscopic retrograde cholangiopancreatography; endoscopic ultrasound-guided fine needle aspiration; endoscopic ultrasound-guided fine needle biopsy; endoscopic ultrasound-guided tissue acquisition; histology; liquid-based cytology; pancreatic ductal adenocarcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
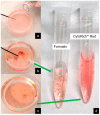


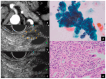
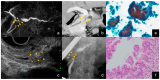
References
-
- Itonaga M., Yasukawa S., Fukutake N., Ogura T., Asada M., Shimokawa T., Inatomi O., Nakai Y., Shiomi H., Nebiki H., et al. Comparison of 22-gauge Standard and Franseen Needles in EUS-guided Tissue Acquisition for Diagnosing Solid Pancreatic Lesions: A Multicenter Randomized Controlled Trial. Gastrointest. Endosc. 2022;96:57–66.e2. doi: 10.1016/j.gie.2022.02.005. - DOI - PubMed
-
- Kandel P., Nassar A., Gomez V., Raimondo M., Woodward T.A., Crook J.E., Fares N.S., Wallace M.B. Comparison of Endoscopic Ultrasound-guided Fine-needle Biopsy Versus Fine-needle Aspiration for Genomic Profiling and DNA Yield in Pancreatic Cancer: A Randomized Crossover Trial. Endoscopy. 2021;53:376–382. doi: 10.1055/a-1223-2171. - DOI - PubMed
-
- Hisada Y., Hijioka S., Ikeda G., Maehara K., Hashimoto T., Kitamura H., Harai S., Yoshinari M., Kawasaki Y., Murashima Y., et al. Proportion of Unresectable Pancreatic Cancer Specimens Obtained by Endoscopic Ultrasound-guided Tissue Acquisition Meeting the Oncoguide™ NCC Oncopanel System Analysis Suitability Criteria: A Single-arm, Phase II Clinical Trial. J. Gastroenterol. 2022;57:990–998. doi: 10.1007/s00535-022-01926-z. - DOI - PubMed
-
- Kawamura R., Ishii Y., Serikawa M., Tsuboi T., Tsushima K., Nakamura S., Hirano T., Ikemoto J., Kiyoshita Y., Saeki S., et al. Optimal Indication of Endoscopic Retrograde Pancreatography-based Cytology in the Preoperative Pathological Diagnosis of Pancreatic Ductal Adenocarcinoma. Pancreatology. 2022;22:414–420. doi: 10.1016/j.pan.2022.02.001. - DOI - PubMed
-
- Crinò S.F., Conti Bellocchi M.C., Bernardoni L., Manfrin E., Parisi A., Amodio A., De Pretis N., Frulloni L., Gabbrielli A. Diagnostic Yield of EUS-FNA of Small (≤15 mm) Solid Pancreatic Lesions Using a 25-gauge Needle. Hepatobiliary Pancreat. Dis. Int. 2018;17:70–74. doi: 10.1016/j.hbpd.2018.01.010. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

