Ultralow Prostate-Specific Antigen (PSA) Levels and Improved Oncological Outcomes in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) Patients Treated with Apalutamide: A Real-World Multicentre Study
- PMID: 39458170
- PMCID: PMC11508498
- DOI: 10.3390/jcm13206221
Ultralow Prostate-Specific Antigen (PSA) Levels and Improved Oncological Outcomes in Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) Patients Treated with Apalutamide: A Real-World Multicentre Study
Abstract
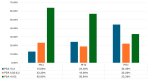
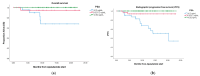
Background/Objectives: Androgen receptor-targeted agents have significantly improved the prognosis of metastatic hormone-sensitive prostate cancer (mHSPC). Prostate-specific antigen (PSA) levels are key prognostic markers, with rapid and deep reductions associated with better outcomes. This study aims to assess the association between the new PSA cut-offs and survival in mHSPC patients treated with Apalutamide. Methods: We conducted a multicentre, retrospective analysis of mHSPC patients treated with Apalutamide between March 2021 and January 2023. Overall survival (OS) and radiographic progression-free survival (rFPS) were analyzed and stratified by the following PSA ranges: <0.02 ng/mL (ultralow), 0.02-0.2 ng/mL, and >0.2 ng/mL. Cox regression was applied to identify variables associated with OS and rPFS. Results: Among 193 patients, 34.2% had de novo mHSPC, with the majority classified as M1b. A total of 58.2% (110) of our cohort achieved ultralow PSA levels, with 20.6% between 0.02 and 0.2 ng/mL, and 21.2% of PSA levels > 0.2 ng/mL. Most patients reached ultralow PSA within six months. Low-volume, metachronous, and M1a subgroups displayed a higher prevalence of patients reaching ultralow PSA levels. At 18 months, OS was 100% in the ultralow PSA group, 94.4% for the 0.02-0.2 ng/mL group, and 67.7% in the >0.2 ng/mL group. Similarly, rPFS at 18 months was 100%, 93.5%, and 50.7%, respectively. Cox regression revealed that both ultralow PSA levels and ISUP grade had a significant impact on OS (HR of 8.256 and 0.164, respectively). For rPFS, only ultralow PSA levels had a significant impact (HR = 0.085). Conclusions: This real-world study of mHSPC patients treated with Apalutamide plus ADT revealed that achieving ultralow PSA levels is strongly associated with better oncological outcomes.
Keywords: PSA levels; metastatic hormone-sensitive prostate cancer; metastatic volume; prostate cancer; ultralow PSA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

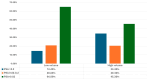
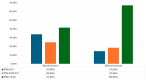
References
-
- Armstrong A.J., Szmulewitz R.Z., Petrylak D.P., Holzbeierlein J., Villers A., Azad A., Alcaraz A., Alekseev B., Iguchi T., Shore N.D., et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

