Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus
- PMID: 39458191
- PMCID: PMC11508752
- DOI: 10.3390/jcm13206241
Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus
Abstract
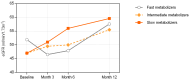
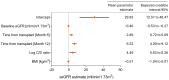
Background: The tacrolimus concentration-to-dose (C/D) ratio is valuable for optimizing nephrotoxicity-related renal outcomes. Prospective data on the C/D ratio in kidney transplant recipients newly treated with MeltDose tacrolimus are limited. We analyzed the C/D ratio pattern of MeltDose tacrolimus and its effect on posttransplant renal function, comparing it with the literature data on immediate-release tacrolimus (IR-Tac). Methods: In total, 101 adult kidney transplant recipients on a standard immunosuppressive regimen including MeltDose tacrolimus were enrolled in this prospective, multicenter cohort study and followed for 12 months. The C/D ratio classified patients as fast, intermediate, or slow metabolizers. Renal function was assessed via the estimated glomerular filtration rate (eGFR). MeltDose tacrolimus data were compared with previous IR-Tac data by bootstrapping. Results: The cohort exhibited a right-skewed C/D ratio distribution with a mean of 2.12 ng/mL × 1/mg, which was significantly greater than the 1.29 mean for IR-Tac (p < 0.001). Compared with fast metabolizers, slow metabolizers of MeltDose tacrolimus experienced greater eGFR gains at 6 months post-transplantation (median +7.9 vs. -3.6 mL/min; p = 0.005). A Bayesian linear mixed-effects model predicting the eGFR at month 12 identified the baseline eGFR, time from transplant, body mass index, and log-transformed C/D ratio as significant variables. A one-unit increase in the log-transformed C/D ratio corresponded to an approximate increase of 4.5 mL/min in the eGFR at month 12. Conclusions: Slow metabolizers of MeltDose tacrolimus had significantly better renal function outcomes than fast metabolizers. MeltDose tacrolimus is associated with slower metabolism than is IR-Tac, as evidenced by its higher C/D ratios.
Keywords: C/D ratio; LCPT; MeltDose; glomerular filtration rate; kidney transplantation; pharmacokinetics; tacrolimus.
Conflict of interest statement
A.D.-Ś. and I.K.-G. received honoraria from Chiesi Poland for lecturing and participation on advisory boards. R.H. is employed by Chiesi Poland, the sponsor of the study. The remaining co-authors declare no conflicts of interest.
Figures

References
-
- Tremblay S., Nigro V., Weinberg J., Woodle E.S., Alloway R.R. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017;17:432–442. doi: 10.1111/ajt.13935. - DOI - PMC - PubMed
-
- Giral M., Grimbert P., Morin B., Bouvier N., Buchler M., Dantal J., Garrigue V., Bertrand D., Kamar N., Malvezzi P., et al. Impact of Switching From Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study. Transpl. Int. 2024;37:11571. doi: 10.3389/ti.2024.11571. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

