Novel Agrobacterium fabrum str. 1D1416 for Citrus Transformation
- PMID: 39458308
- PMCID: PMC11509345
- DOI: 10.3390/microorganisms12101999
Novel Agrobacterium fabrum str. 1D1416 for Citrus Transformation
Abstract
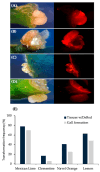
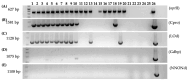
Citrus is one of the world's most important and widely produced fruit crops, with over a 100 million metric tons harvested from nearly 10 million hectares in 2023. Challenges in crop maintenance, production, and fruit quality necessitate developing new traits through Agrobacterium-mediated genetic transformation. While a few Agrobacterium strains (EHA105, GV3101, LBA4404) are known to transform citrus, many wild strains remain untested. We screened forty-one wild-type Agrobacterium strains isolated from various woody species and identified five capable of DNA transfer into citrus cells. Strain 1D1416 demonstrated the highest transient transformation frequency in Carrizo epicotyl explants (88%), outperforming the control EHA105 (84%) with comparable shoot regeneration rates (32% and 42%, respectively). Notably, 1D1416 exhibited no overgrowth and had the lowest necrosis and mortality rates in transformed tissues. It efficiently transferred the DsRed gene and induced galls in mature tissues of Mexican lime (70%), lemon (48%), Washington navel orange (25%), and clementine (6%). Genome sequencing of 1D1416 allowed for the disarming of the native T-DNA and addition of GAANTRY technology. This novel strain, combined with an optimized transformation procedure, make it a valuable tool for advancing citrus transformation.
Keywords: Agrobacterium rhizogenes; Agrobacterium tumefaciens; GAANTRY system; citrus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Malpighi M. Anatome plantarum. Cui Subjungjungitur Appendix, Iteratas & Auctas Ejusdem Authoris de ovo Incubato Observationes Continens. Regiae Societati, Londini ad Scientan Naturalem Promovendam Institutae, Dicata. J. Martyn; London, UK: 1675.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

