Inhibition of Angiogenesis and Effect on Inflammatory Bowel Disease of Ginsenoside Rg3-Loaded Thermosensitive Hydrogel
- PMID: 39458575
- PMCID: PMC11509886
- DOI: 10.3390/pharmaceutics16101243
Inhibition of Angiogenesis and Effect on Inflammatory Bowel Disease of Ginsenoside Rg3-Loaded Thermosensitive Hydrogel
Abstract
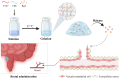
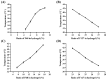
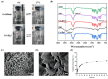
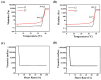
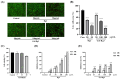
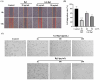
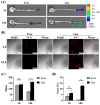
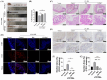
Background: Inflammatory bowel disease (IBD), characterized by chronic inflammation of the digestive tract, involves angiogenesis as a key pathogenic mechanism. Ginsenoside Rg3, derived from the traditional Chinese herb ginseng, is recognized for its anti-angiogenic properties but is limited by low oral bioavailability. This necessitates the development of an alternative delivery system to improve its therapeutic effectiveness. Methods: Pluronic F-127 (F127) and Pluronic F-68 (F68) were used to construct Rg3-loaded thermosensitive hydrogel Gel-Rg3. Meanwhile, a series of physicochemical properties were determined. Then the safety and pharmacological activity of Gel-Rg3 were evaluated in vitro and in vivo using human umbilical vein endothelial cells (HUVECs) and colitis mouse model, in order to initially validate the potential of Gel-Rg3 for the treatment of IBD. Results: We engineered a rectally administrable, thermosensitive Gel-Rg3 hydrogel using F127 and F68, which forms at body temperature, enhancing Rg3's intestinal retention and slowly releasing the drug. In vitro, Gel-Rg3 demonstrated superior anti-angiogenic activity by inhibiting HUVEC proliferation, migration, and tube formation. It also proved safer and better suited for IBD's delicate intestinal environment than unformulated Rg3. In vivo assessments confirmed increased intestinal adhesion and anti-angiogenic efficacy. Conclusions: The Gel-Rg3 hydrogel shows promise for IBD therapy by effectively inhibiting angiogenesis via rectal delivery, overcoming Rg3's bioavailability limitations with improved safety and efficacy. This study provides new inspiration and data support for the design of treatment strategies for IBD.
Keywords: angiogenesis; drug delivery system; ginsenoside Rg3; inflammatory bowel disease; thermosensitive hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Poloxamer 407 and Hyaluronic Acid Thermosensitive Hydrogel-Encapsulated Ginsenoside Rg3 to Promote Skin Wound Healing.Front Bioeng Biotechnol. 2022 Jul 5;10:831007. doi: 10.3389/fbioe.2022.831007. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35866029 Free PMC article.
-
Evaluation of 20(S)-ginsenoside Rg3 loaded hydrogel for the treatment of perianal ulcer in a rat model.J Ginseng Res. 2022 Nov;46(6):771-779. doi: 10.1016/j.jgr.2022.03.002. Epub 2022 Mar 11. J Ginseng Res. 2022. PMID: 36312740 Free PMC article.
-
Rg3-loaded P407/CS/HA hydrogel inhibits UVB-induced oxidative stress, inflammation and apoptosis in HaCaT cells.Biomed Pharmacother. 2023 Sep;165:115177. doi: 10.1016/j.biopha.2023.115177. Epub 2023 Jul 17. Biomed Pharmacother. 2023. PMID: 37467650
-
The protective role of ginsenoside Rg3 in heart diseases and mental disorders.Front Pharmacol. 2024 Feb 26;15:1327033. doi: 10.3389/fphar.2024.1327033. eCollection 2024. Front Pharmacol. 2024. PMID: 38469409 Free PMC article. Review.
-
Anti-Angiogenic Properties of Ginsenoside Rg3.Molecules. 2020 Oct 23;25(21):4905. doi: 10.3390/molecules25214905. Molecules. 2020. PMID: 33113992 Free PMC article. Review.
Cited by
-
Unlocking ginsenosides' therapeutic power with polymer-based delivery systems: current applications and future perspectives.Front Pharmacol. 2025 Jul 10;16:1629803. doi: 10.3389/fphar.2025.1629803. eCollection 2025. Front Pharmacol. 2025. PMID: 40709093 Free PMC article. Review.
-
Angiogenic Factors and Inflammatory Bowel Diseases.Biomedicines. 2025 May 9;13(5):1154. doi: 10.3390/biomedicines13051154. Biomedicines. 2025. PMID: 40426981 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

