Buprenorphine Transdermal Delivery System: Bioequivalence Assessment and Adhesion Performance of Two Patch Formulations
- PMID: 39458581
- PMCID: PMC11510105
- DOI: 10.3390/pharmaceutics16101249
Buprenorphine Transdermal Delivery System: Bioequivalence Assessment and Adhesion Performance of Two Patch Formulations
Abstract
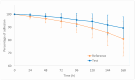
Background and Objective: Buprenorphine is an opioid drug indicated for the management of severe and persistent pain. The buprenorphine transdermal patch provides a non-invasive method of rate-controlled drug release, ensuring constant and predictable drug plasma levels over an extended period. This study aimed to assess the bioequivalence, skin adhesion non-inferiority, and tolerability of two buprenorphine transdermal patches to meet the regulatory requirements for the registration of a generic product in Brazil. Methods: A randomized, single-dose, two-period, two-sequence crossover trial was performed involving healthy subjects of both genders. The subjects received a single dose of either the test formulation or the reference formulation (Restiva®), separated by a 29-day washout period. For pharmacokinetic analysis, blood samples were collected up to 12 days post-dose and quantified using a validated bioanalytical method. Skin adhesion was assessed over a 7-day period (dosing interval) following patch application. Seventy-six subjects were enrolled and fifty-two completed the study. Results and Conclusion: The 90% confidence intervals for Cmax, AUC0-t, and partial AUCs were within the acceptable bioequivalence limits of 80 to 125%. Adhesion comparison showed the non-inferiority of the test formulation. Based on ANVISA's regulatory requirements, the test and reference formulations were considered bioequivalent and could be interchangeable in clinical practice.
Keywords: adhesion; bioequivalence; buprenorphine; opioid; patch; pharmacokinetics; transdermal delivery system.
Conflict of interest statement
The authors M.F., A.S., L.d.S.T., K.B.B. and A.C.C.S. declare no conflicts of interest regarding the publication of this article. The authors M.G.D., J.M., F.C., T.M.d.S., D.R.B.V. and C.F.P.V. are employees of Adium S.A. (sponsor of the study). The authors were fully responsible for all the content and editorial decisions.
Figures


References
-
- Margetts L., Sawyer R. Continuing Education in Anaesthesia Critical Care & Pain. Volume 7. Elsevier; Amsterdam, The Netherlands: 2007. Transdermal drug delivery: Principles and opioid therapy; pp. 171–176. - DOI
-
- De Marco I. Transdermal Patches Containing Opioids in the Treatment of Patients with Chronic Pain. Processes. 2023;11:2673. doi: 10.3390/pr11092673. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

