A Modified Cell-Penetrating Peptide Enhances Insulin and Oxytocin Delivery across an RPMI 2650 Nasal Epithelial Cell Barrier In Vitro
- PMID: 39458599
- PMCID: PMC11510563
- DOI: 10.3390/pharmaceutics16101267
A Modified Cell-Penetrating Peptide Enhances Insulin and Oxytocin Delivery across an RPMI 2650 Nasal Epithelial Cell Barrier In Vitro
Abstract
Background/Objectives: Peptide-based treatments represent an expanding area and require innovative approaches to enhance bioavailability. Combination with cell-penetrating peptides (CPPs) is an attractive strategy to improve non-invasive delivery across nasal epithelial barriers for systemic and direct nose-to-brain transport. We previously developed a modified CPP system termed Glycosaminoglycan-binding Enhanced Transduction (GET) that improves insulin delivery across gastrointestinal epithelium. It contains a membrane docking sequence to promote cellular interactions (P21), a cationic polyarginine domain to stimulate uptake (8R) and an endosomal escaping sequence to maximize availability for onward distribution (LK15). It is synthesized as a single 44-residue peptide (P21-LK15-8R; PLR).
Methods: The current research used in vitro assays for a novel exploration of PLR's ability to improve the transport of two contrasting peptides, insulin (51 residues, net negative charge) and oxytocin (9 residues, weak positive charge) across an RPMI 2650 human nasal epithelial cell barrier cultured at the air-liquid interface.
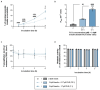
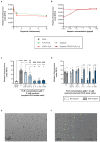
Results: PLR enhanced insulin transcytosis over a 6 h period by 7.8-fold when used at a 2:1 molar ratio of insulin/PLR (p < 0.0001 versus insulin alone). Enhanced oxytocin transcytosis (5-fold) occurred with a 1:10 ratio of oytocin/PLR (p < 0.01). Importantly, these were independent of any impact on transepithelial electrical resistance (TEER) or cell viability (p > 0.05).
Conclusions: We advocate the continued evaluation of insulin-PLR and oxytocin-PLR formulations, including longer-term assessments of ciliotoxicity and cytotoxicity in vitro followed by in vivo assessments of systemic and nose-to-brain delivery.
Keywords: RPMI 2650; cell-penetrating peptide; glycosaminoglycan-GAG-binding enhanced transduction; insulin; nasal drug delivery; oxytocin; transcytosis; transepithelial delivery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Dixon J.E., Osman G., Morris G.E., Markides H., Rotherham M., Bayoussef Z., El Haj A.J., Denning C., Shakesheff K.M. Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell-penetrating peptides. Proc. Natl. Acad. Sci. USA. 2016;113:E291–E299. doi: 10.1073/pnas.1518634113. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

