Synergistic Enhancement of Carboplatin Efficacy through pH-Sensitive Nanoparticles Formulated Using Naturally Derived Boswellia Extract for Colorectal Cancer Therapy
- PMID: 39458611
- PMCID: PMC11510476
- DOI: 10.3390/pharmaceutics16101282
Synergistic Enhancement of Carboplatin Efficacy through pH-Sensitive Nanoparticles Formulated Using Naturally Derived Boswellia Extract for Colorectal Cancer Therapy
Abstract
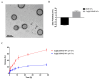
Carboplatin (Cp) is a potent chemotherapeutic agent, but its effectiveness is constrained by its associated side effects. Frankincense, an oleo-gum resin from the Boswellia sacra tree, has demonstrated cytotoxic activity against cancer cells. This study explored the synergistic potential of nanoparticles formulated from Boswellia sacra methanolic extract (BME), to enhance the therapeutic efficacy of Cp at reduced doses. Nanoparticles were prepared via the nanoprecipitation method, loaded with Cp, and coated with positively charged chitosan (CS) for enhanced cell interaction, yielding Cp@CS/BME NPs with an average size of 160.2 ± 4.6 nm and a zeta potential of 12.7 ± 1.5 mV. In vitro release studies revealed a pH-sensitive release profile, with higher release rates at pH 5.4 than at pH 7.4, highlighting the potential for targeted drug delivery in acidic tumor environments. In vitro studies on HT-29 and Caco-2 colorectal cancer cell lines demonstrated the nanoformulation's ability to significantly increase Cp uptake and cytotoxic activity. Apoptosis assays further confirmed increased induction of cell death with Cp@CS/BME NPs. Cell-cycle analysis revealed that treatment with Cp@CS/BME NPs led to a significant increase in the sub-G1 phase, indicative of enhanced apoptosis, and a marked decrease in the G1-phase population coupled with an increased G2/M-phase arrest in both cell lines. Further gene expression analysis demonstrated a substantial downregulation of the anti-apoptotic gene Bcl-2 and an upregulation of the pro-apoptotic genes Bax, PUMA, and BID following treatment with Cp@CS/BME NPs. Thus, this study presents a promising and innovative strategy for enhancing the therapeutic efficacy of chemotherapeutic agents using naturally derived ingredients while limiting the side effects.
Keywords: chemotherapy; colorectal cancer; nanomedicine; nanoprecipitation; pH-sensitive release.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Sedky N.K., Abdel-Kader N.M., Issa M.Y., Abdelhady M.M.M., Shamma S.N., Bakowsky U., Fahmy S.A. Co-Delivery of Ylang Ylang Oil of Cananga odorata and Oxaliplatin Using Intelligent pH-Sensitive Lipid-Based Nanovesicles for the Effective Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023;24:8392. doi: 10.3390/ijms24098392. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

