Leveraging the Aggregated Protein Dye YAT2150 for Malaria Chemotherapy
- PMID: 39458619
- PMCID: PMC11514582
- DOI: 10.3390/pharmaceutics16101290
Leveraging the Aggregated Protein Dye YAT2150 for Malaria Chemotherapy
Abstract
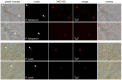
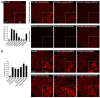
Background/Objectives: YAT2150 is a first-in-class antiplasmodial compound that has been recently proposed as a new interesting drug for malaria therapy. Methods/Results: The fluorescence of YAT2150 rapidly increases upon its entry into Plasmodium, a property that can be of use for the design of highly sensitive diagnostic approaches. YAT2150 blocks the in vitro development of the ookinete stage of Plasmodium and, when added to an infected blood meal, inhibits oocyst formation in the mosquito. Thus, the compound could possibly contribute to future transmission-blocking antimalarial strategies. Cell influx/efflux studies in Caco-2 cells suggest that YAT2150 is internalized by endocytosis and also through the OATP2B1 transporter, whereas its main export route would be via OSTα. YAT2150 has an overall favorable drug metabolism and pharmacokinetics profile, and its moderate cytotoxicity can be significantly reduced upon encapsulation in immunoliposomes, which leads to a dramatic increase in the drug selectivity index to values close to 1000. Although YAT2150 binds amyloid-forming peptides, its in vitro fluorescence emission is stronger upon association with peptides that form amorphous aggregates, suggesting that regions enriched in unstructured proteins are the preferential binding sites of the drug inside Plasmodium cells. The reduction of protein aggregation in the parasite after YAT2150 treatment, which has been suggested to be directly related to the drug's mode of action, is also observed following treatment with quinoline antimalarials like chloroquine and primaquine. Conclusions: Altogether, the data presented here indicate that YAT2150 can represent the spearhead of a new family of compounds for malaria diagnosis and therapy due to its presumed novel mode of action based on the interaction with functional protein aggregates in the pathogen.
Keywords: Plasmodium falciparum; YAT2150; malaria; protein aggregation.
Conflict of interest statement
A patent application (WO 2023/067170 A1; filing date: 21 October 2022) has been filed to protect some of the results presented in this paper, which includes as inventors Inés Bouzón-Arnáiz, Elsa M. Arce, Diego Muñoz-Torrero, and Xavier Fernàndez-Busquets. Authors Benigno Crespo and Sara Viera were employed by the company GlaxoSmithKline. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Phyo A.P., Ashley E.A., Anderson T.J.C., Bozdech Z., Carrara V.I., Sriprawat K., Nair S., White M.M., Dziekan J., Ling C., et al. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): The role of parasite genetic factors. Clin. Infect. Dis. 2016;63:784–791. doi: 10.1093/cid/ciw388. - DOI - PMC - PubMed
-
- Uwimana A., Legrand E., Stokes B.H., Ndikumana J.M., Warsame M., Umulisa N., Ngamije D., Munyaneza T., Mazarati J.B., Munguti K., et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020;26:1602–1608. doi: 10.1038/s41591-020-1005-2. - DOI - PMC - PubMed
-
- Mathieu L.C., Cox H., Early A.M., Mok S., Lazrek Y., Paquet J.C., Ade M.P., Lucchi N.W., Grant Q., Udhayakumar V., et al. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. eLife. 2020;9:e51015. doi: 10.7554/eLife.51015. - DOI - PMC - PubMed
Grants and funding
- PID2021-128325OB-I00/Ministerio de Ciencia, Innovación y Universidades
- PDC2022-133085-I00/Ministerio de Ciencia, Innovación y Universidades
- PID2020-118127RB-I00/Ministerio de Ciencia, Innovación y Universidades
- PID2021-124765OB-I00/Ministerio de Ciencia, Innovación y Universidades
- HR17-00409/CaixaBank
LinkOut - more resources
Full Text Sources

