A Human Brain-Chip for Modeling Brain Pathologies and Screening Blood-Brain Barrier Crossing Therapeutic Strategies
- PMID: 39458643
- PMCID: PMC11510380
- DOI: 10.3390/pharmaceutics16101314
A Human Brain-Chip for Modeling Brain Pathologies and Screening Blood-Brain Barrier Crossing Therapeutic Strategies
Abstract
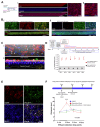
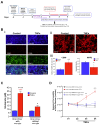
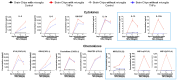
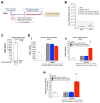
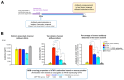
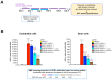
Background/Objectives: The limited translatability of preclinical experimental findings to patients remains an obstacle for successful treatment of brain diseases. Relevant models to elucidate mechanisms behind brain pathogenesis, including cell-specific contributions and cell-cell interactions, and support successful targeting and prediction of drug responses in humans are urgently needed, given the species differences in brain and blood-brain barrier (BBB) functions. Human microphysiological systems (MPS), such as Organ-Chips, are emerging as a promising approach to address these challenges. Here, we examined and advanced a Brain-Chip that recapitulates aspects of the human cortical parenchyma and the BBB in one model. Methods: We utilized human primary astrocytes and pericytes, human induced pluripotent stem cell (hiPSC)-derived cortical neurons, and hiPSC-derived brain microvascular endothelial-like cells and included for the first time on-chip hiPSC-derived microglia. Results: Using Tumor necrosis factor alpha (TNFα) to emulate neuroinflammation, we demonstrate that our model recapitulates in vivo-relevant responses. Importantly, we show microglia-derived responses, highlighting the Brain-Chip's sensitivity to capture cell-specific contributions in human disease-associated pathology. We then tested BBB crossing of human transferrin receptor antibodies and conjugated adeno-associated viruses. We demonstrate successful in vitro/in vivo correlation in identifying crossing differences, underscoring the model's capacity as a screening platform for BBB crossing therapeutic strategies and ability to predict in vivo responses. Conclusions: These findings highlight the potential of the Brain-Chip as a reliable and time-efficient model to support therapeutic development and provide mechanistic insights into brain diseases, adding to the growing evidence supporting the value of MPS in translational research and drug discovery.
Keywords: cell-specific contributions; microfluidics brain-chip; physiologically relevant responses; screening of BBB-crossing therapeutics.
Conflict of interest statement
S.M.C., K.H. and B.Z. are full-time employees of Regeneron Pharmaceuticals Inc. and receive stock options/restricted stock as part of their compensation. K.K. and E.P. were full-time employees of Regeneron Pharmaceuticals Inc. and received stock options/restricted stock as part of their compensation during their employment.
Figures
References
-
- Atkins J.T., George G.C., Hess K., Marcelo-Lewis K.L., Yuan Y., Borthakur G., Khozin S., LoRusso P., Hong D.S. Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials. Br. J. Cancer. 2020;123:1496–1501. doi: 10.1038/s41416-020-01033-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

