Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering
- PMID: 39458681
- PMCID: PMC11511575
- DOI: 10.3390/polym16202853
Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering
Abstract
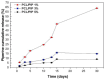
Tissue engineering has great potential for the restoration of damaged tissue due to injury or disease. During tissue development, scaffolds provide structural support for cell growth. To grow healthy tissue, the principal components of such scaffolds must be biocompatible and nontoxic. Poly(ε-caprolactone) (PCL) is a biopolymer that has been used as a key component of composite scaffolds for tissue engineering applications due to its mechanical strength and biodegradability. However, PCL alone can have low cell adherence and wettability. Blends of biomaterials can be incorporated to achieve synergistic scaffold properties for tissue engineering. Electrospun PCL-based scaffolds consist of single or blended-composition nanofibers and nanofibers with multi-layered internal architectures (i.e., core-shell nanofibers or multi-layered nanofibers). Nanofiber diameter, composition, and mechanical properties, biocompatibility, and drug-loading capacity are among the tunable properties of electrospun PCL-based scaffolds. Scaffold properties including wettability, mechanical strength, and biocompatibility have been further enhanced with scaffold layering, surface modification, and coating techniques. In this article, we review nanofibrous electrospun PCL-based scaffold fabrication and the applications of PCL-based scaffolds in tissue engineering as reported in the recent literature.
Keywords: biocompatibility; biomaterials; composite scaffolds; electrospinning; nanofibers; poly(ε-caprolactone) (PCL); scaffold fabrication; scaffold wettability; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Guimarães C.F., Gasperini L., Marques A.P., Reis R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020;5:351–370. doi: 10.1038/s41578-019-0169-1. - DOI
-
- Mu J., Luo D., Li W., Ding Y. Multiscale Polymeric Fibers for Drug Delivery and Tissue Engineering. Biomed. Technol. 2024;5:60–72. doi: 10.1016/j.bmt.2023.05.001. - DOI
-
- Diedkova K., Pogrebnjak A.D., Kyrylenko S., Smyrnova K., Buranich V.V., Horodek P., Zukowski P., Koltunowicz T.N., Galaszkiewicz P., Makashina K., et al. Polycaprolactone-Mxene Nanofibrous Scaffolds for Tissue Engineering. ACS Appl. Mater. Interfaces. 2023;15:14033–14047. doi: 10.1021/acsami.2c22780. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

