Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking
- PMID: 39458712
- PMCID: PMC11511556
- DOI: 10.3390/polym16202884
Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking
Abstract
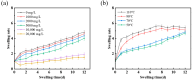
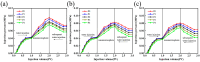
Urethane surfactants (REQ) were synthesized with octadecanol ethoxylate (AEO) and isocyanate methacrylate (IEM). Subsequently, reactive-carbamate-surfactant-modified nanomicrospheres (PER) were prepared via two-phase aqueous dispersion polymerization using acrylamide (AM), 2-acrylamido-2-methylpropanesulfonic acid (AMPS) and ethylene glycol dimethacrylate (EGDMA). The microstructures and properties of the nanomicrospheres were characterized and examined via infrared spectroscopy, nano-laser particle size analysis, scanning electron microscopy, and in-house simulated exfoliation experiments. The results showed that the synthesized PER nanomicrospheres had a uniform particle size distribution, with an average size of 336 nm. The thermal decomposition temperature of the nanomicrospheres was 278 °C, and the nanomicrospheres had good thermal stability. At the same time, the nanomicrospheres maintained good swelling properties at mineralization < 10,000 mg/L and temperature < 90 °C. Under the condition of certain permeability, the blocking rate and drag coefficient gradually increased with increasing polymer microsphere concentration. Furthermore, at certain polymer microsphere concentrations, the blocking rate and drag coefficient gradually decreased with increasing core permeability. The experimental results indicate that nanomicrospheres used in the artificial core simulation drive have a better ability to drive oil recovery. Compared with AM microspheres (without REQ modification), nanomicrospheres exert a more considerable effect on recovery improvement. Compared with the water drive stage, the final recovery rate after the drive increases by 23.53%. This improvement is attributed to the unique structural design of the nanorods, which can form a thin film at the oil-water-rock interface and promote oil emulsification and stripping. In conclusion, PER nanomicrospheres can effectively control the fluid dynamics within the reservoir, reduce the loss of oil and gas resources, and improve the economic benefits of oil and gas fields, giving them a good application prospect.
Keywords: blocking rate; moderator; nanomicrospheres; recovery rate; surfactant modified.
Conflict of interest statement
Author Huaqiang Shi was employed by the company Oil & Gas Technology Research Institute of Changqing Oilfield Branch Company, PetroChina. Author Haibin Li and Jiali Chen were employed by the company Xi’an Wonder Energy Chemical Co., Ltd. Author Yulong Li was employed by the company Shaanxi Rixin Petrochemical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Baffie F., Patias G., Shegiwal A., Brunel F., Monteil V., Verrieux L., Perrin L., Haddleton D.M., Dagosto F. Block Copolymers Based on Ethylene and Methacrylates Using a Combination of Catalytic Chain Transfer Polymerisation (CCTP) and Radical Polymerisation, Angewandte Chemie International Edition. Angew. Chem. Int. Ed. 2021;133:25560–25568. doi: 10.1002/ange.202108996. - DOI - PMC - PubMed
-
- Preusser C., Chovancova A., Lacik L., Hutchinson R. Modeling the Radical Batch Homopolymerization ofAcrylamide in Aqueous Solution. Macromol. React. Eng. 2016;10:490–501. doi: 10.1002/mren.201500076. - DOI
-
- Giz A., Giz-Catalgil H., Alb A., Brousseau J.L., Reed W. Kinetics and Mechanisms of Acrvlamide Polymerization from Absolute, Online Monitoring of Polymerization Reaction. Macromolecules. 2001;34:1180–1191. doi: 10.1021/ma000815s. - DOI
-
- Zhou Y.N., Li J.J., Wang T.T., Wu Y.Y., Luo H.Z. Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering. Prog. Polym. Sci. 2022;130:101555. doi: 10.1016/j.progpolymsci.2022.101555. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

