Phenylethynyl-Terminated Imide Oligomer-Based Thermoset Resins
- PMID: 39458775
- PMCID: PMC11511106
- DOI: 10.3390/polym16202947
Phenylethynyl-Terminated Imide Oligomer-Based Thermoset Resins
Abstract
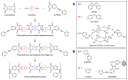
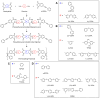
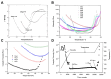
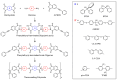
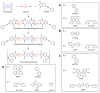
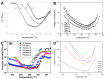
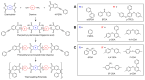
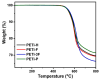
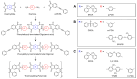
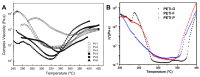
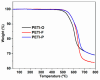
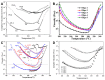
Phenylethynyl-terminated imide (PETI) oligomers are highly valued for their diverse applications in films, moldings, adhesives, and composite material matrices. PETIs can be synthesized at varying molecular weights, enabling the fine-tuning of their properties to meet specific application requirements. Upon thermal curing, these oligomers form super-rigid network structures that enhance solvent resistance, increase glass-transition temperatures, and improve elastic moduli. Their low molecular weights and melt viscosities further facilitate processing, making them particularly suitable for composites and adhesive bonding. This review examines recent advancements in developing ultra-high-temperature PETIs, focusing on their structure-processing-properties relationships. It begins with an overview of the historical background and key physicochemical characteristics of PETIs, followed by a detailed discussion of PETIs synthesized from monomers featuring noncoplanar configurations (including kink and cardo structures), fluorinated groups, flexible linkages, and liquid crystalline mesogenic structures. The review concludes by addressing current challenges in this research field and exploring potential future directions.
Keywords: composite material matrix; phenylethynyl-terminated imide oligomer; structure parameters; thermoset resin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Hergenrother P.M., Smith J.G. Chemistry and Properties of Imide Oligomers End-Capped with Phenylethynylphthalic Anhydrides. Polymer. 1994;35:4857–4864. doi: 10.1016/0032-3861(94)90744-7. - DOI
-
- Connell J.W., Smith J.G., Hergenrother P.M. Oligomers and Polymers Containing Phenylethynyl Groups. J. Macromol. Sci. Part C Polym. Rev. 2000;40:207–230. doi: 10.1081/MC-100100585. - DOI
-
- Yokota R., Yamamoto S., Yano S., Sawaguchi T., Hasegawa M., Yamaguchi H., Ozawa H., Sato R. Molecular Design of Heat Resistant Polyimides Having Excellent Processability and High Glass Transition Temperature. High Perform. Polym. 2001;13:S61–S72. doi: 10.1088/0954-0083/13/2/306. - DOI
-
- Cho D., Drzal L.T. Characterization, Properties, and Processing of LaRCTM PETI-5 as a High-Temperature Sizing Material. I. FTIR Studies on Imidization and Phenylethynyl End-Group Reaction Behavior. J. Appl. Polym. Sci. 2000;76:190–200. doi: 10.1002/(SICI)1097-4628(20000411)76:2<190::AID-APP8>3.0.CO;2-8. - DOI
-
- Fang X., Xie X., Simone C.D., Stevens M.P., Scola D.A. A Solid-State 13C NMR Study of the Cure of 13C-Labeled Phenylethynyl End-Capped Polyimides. Macromolecules. 2000;33:1671–1681. doi: 10.1021/ma991197m. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

