Genome-Wide Transcriptional Response of Avocado to Fusarium sp. Infection
- PMID: 39458832
- PMCID: PMC11511450
- DOI: 10.3390/plants13202886
Genome-Wide Transcriptional Response of Avocado to Fusarium sp. Infection
Abstract
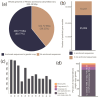
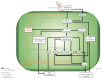
The avocado crop is relevant for its economic importance and because of its unique evolutionary history. However, there is a lack of information regarding the molecular processes during the defense response against fungal pathogens. Therefore, using a genome-wide approach in this work, we investigated the transcriptional response of the Mexican horticultural race of avocado (Persea americana var. drymifolia), including miRNAs profile and their possible targets. For that, we established an avocado-Fusarium hydroponic pathosystem and studied the response for 21 days. To guarantee robustness in the analysis, first, we improved the avocado genome assembly available for this variety, resulting in 822.49 Mbp in length with 36,200 gene models. Then, using an RNA-seq approach, we identified 13,778 genes differentially expressed in response to the Fusarium infection. According to their expression profile across time, these genes can be clustered into six groups, each associated with specific biological processes. Regarding non-coding RNAs, 8 of the 57 mature miRNAs identified in the avocado genome are responsive to infection caused by Fusarium, and the analysis revealed a total of 569 target genes whose transcript could be post-transcriptionally regulated. This study represents the first research in avocados to comprehensively explore the role of miRNAs in orchestrating defense responses against Fusarium spp. Also, this work provides valuable data about the genes involved in the intricate response of the avocado during fungal infection.
Keywords: Fusarium infection; Persea americana; defense response; miRNAs.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Camargo Ramírez R.d.C. Ph.D. Thesis. Universitat Autònoma de Barcelona; Barcelona, Spain: 2017. Function of microRNAs in Plant Innate Immunity.
-
- Pandey R., Bhardwaj A.R., Agarwal M., Katiyar-Agarwal S. Discovery of small RNAs in wheat: A survey. Indian J. Plant Physiol. 2017;22:411–421. doi: 10.1007/s40502-017-0338-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

