Ascophyllum nodosum Extract Improves Olive Performance Under Water Deficit Through the Modulation of Molecular and Physiological Processes
- PMID: 39458857
- PMCID: PMC11511455
- DOI: 10.3390/plants13202908
Ascophyllum nodosum Extract Improves Olive Performance Under Water Deficit Through the Modulation of Molecular and Physiological Processes
Abstract
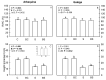
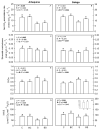
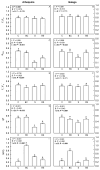
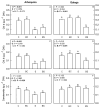
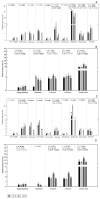
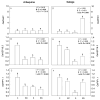
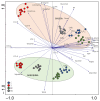
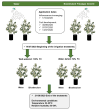
The olive tree is well adapted to the Mediterranean climate, but how orchards based on intensive practices will respond to increasing drought is unknown. This study aimed to determine if the application of a commercial biostimulant improves olive tolerance to drought. Potted plants (cultivars Arbequina and Galega) were pre-treated with an extract of Ascophyllum nodosum (four applications, 200 mL of 0.50 g/L extract per plant), and were then well irrigated (100% field capacity) or exposed to water deficit (50% field capacity) for 69 days. Plant height, photosynthesis, water status, pigments, lipophilic compounds, and the expression of stress protective genes (OeDHN1-protective proteins' dehydrin; OePIP1.1-aquaporin; and OeHSP18.3-heat shock proteins) were analyzed. Water deficit negatively affected olive physiology, but the biostimulant mitigated these damages through the modulation of molecular and physiological processes according to the cultivar and irrigation. A. nodosum benefits were more expressive under water deficit, particularly in Galega, promoting height (increase of 15%) and photosynthesis (increase of 34%), modulating the stomatal aperture through the regulation of OePIP1.1 expression, and keeping OeDHN1 and OeHSP18.3 upregulated to strengthen stress protection. In both cultivars, biostimulant promoted carbohydrate accumulation and intrinsic water-use efficiency (iWUE). Under good irrigation, biostimulant increased energy availability and iWUE in Galega. These data highlight the potential of this biostimulant to improve olive performance, providing higher tolerance to overcome climate change scenarios. The use of this biostimulant can improve the establishment of younger olive trees in the field, strengthen the plant's capacity to withstand field stresses, and lead to higher growth and crop productivity.
Keywords: aquaporins; biostimulants; dehydrins; drought; heat shock proteins; photosynthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Harris R.M.B., Loeffler F., Rumm A., Fischer C., Horchler P., Scholz M., Foeckler F., Henle K. Biological responses to extreme weather events are detectable but difficult to formally attribute to anthropogenic climate change. Sci. Rep. 2020;10:14067. doi: 10.1038/s41598-020-70901-6. - DOI - PMC - PubMed
-
- Fraga H., Moriondo M., Leolini L., Santos J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy. 2021;11:56. doi: 10.3390/agronomy11010056. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

