Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice
- PMID: 39458934
- PMCID: PMC11510413
- DOI: 10.3390/ph17101293
Alleviation of Autophagic Deficits and Neuroinflammation by Histamine H3 Receptor Antagonist E159 Ameliorates Autism-Related Behaviors in BTBR Mice
Abstract
Background/objectives: Autism spectrum disorder (ASD) is a neurodevelopmental condition marked by social interaction difficulties, repetitive behaviors, and immune dysregulation with elevated pro-inflammatory markers. Autophagic deficiency also contributes to social behavior deficits in ASD. Histamine H3 receptor (H3R) antagonism is a potential treatment strategy for brain disorders with features overlapping ASD, such as schizophrenia and Alzheimer's disease.
Methods: This study investigated the effects of sub-chronic systemic treatment with the H3R antagonist E159 on social deficits, repetitive behaviors, neuroinflammation, and autophagic disruption in male BTBR mice.
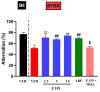
Results: E159 (2.5, 5, and 10 mg/kg, i.p.) improved stereotypic repetitive behavior by reducing self-grooming time and enhancing spontaneous alternation in addition to attenuating social deficits. It also decreased pro-inflammatory cytokines in the cerebellum and hippocampus of treated BTBR mice. In BTBR mice, reduced expression of autophagy-related proteins LC3A/B and Beclin 1 was observed, which was elevated following treatment with E159, attenuating the disruption in autophagy. The co-administration with the H3R agonist MHA (10 mg/kg, i.p.) reversed these effects, highlighting the role of histaminergic neurotransmission in observed behavioral improvements.
Conclusions: These preliminary findings suggest the therapeutic potential of H3R antagonists in targeting neuroinflammation and autophagic disruption to improve ASD-like behaviors.
Keywords: BTBR mice; H3 receptor antagonists; autism spectrum disorder; autophagy; mTOR; neuroinflammation; repetitive behavior; social features.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Dana H., Tahtasakal R., Sener E.F. Animal Models of Autism: A Perspective from Autophagy Mechanisms. J. Transl. Genet. Genom. 2020;4:251–262. doi: 10.20517/jtgg.2020.25. - DOI
-
- Jiang P., Zhou L., Zhao L., Fei X., Wang Z., Liu T., Tang Y., Li D., Gong H., Luo Y., et al. Puerarin Attenuates Valproate-Induced Features of ASD in Male Mice via Regulating Slc7a11-Dependent Ferroptosis. Neuropsychopharmacology. 2024;49:497–507. doi: 10.1038/s41386-023-01659-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

