Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds
- PMID: 39458999
- PMCID: PMC11509960
- DOI: 10.3390/ph17101358
Vesicle-Transported Multidrug Resistance as a Possible Therapeutic Target of Natural Compounds
Abstract
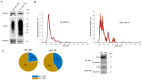
Background/objectives: A key role of extracellular vesicles (EVs) is mediating both cell-cell and cell-stroma communication in pathological/physiological conditions. EVs from resistant tumor cells can transport different molecules like P-glycoprotein (P-gp), acting as a shuttle between donor and recipient cells, resulting in a phenotypic change. The aim of our work was to isolate, characterize, and inhibit the release of EVs in two multidrug resistance (MDR) cancer models: MCF-7R (breast cancer cell line) and HL-60R (acute myeloid leukemia cell line).
Methods: The existence of P-gp in EVs from MDR cells was confirmed by Western blotting assays. The characterization of EVs was carried out by evaluating the size using NTA and the presence of specific markers such as CD63, Hsp70 and Syntenin. The ability of HL-60R and MCF-7R to perform horizontal transfer of P-gp via EVs to sensitive cells was assessed using three different methods. The acquisition of resistance and its inhibition in recipient cells was confirmed by MTS 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay.
Results: Our data showed that cell lines (MDR) release P-gp-loaded EVs, unlike sensitive cells. The acquisition of resistance determined by the incorporation of P-gp into the membrane of sensitive cells was confirmed by the reduced cytotoxic activity of doxorubicin. Natural compounds such as curcumin, lupeol, and heptacosane can block vesicular transfer and restore the sensitivity of HL-60 and MCF-7 cells.
Conclusions: Our study demonstrates that natural inhibitors able to reverse this mechanism may represent a new therapeutic strategy to limit the propagation of the resistant phenotype.
Keywords: P-glycoprotein; acute myeloid leukemia; breast cancer; cancer drug resistance; extracellular vesicles.
Conflict of interest statement
All authors declare there are no conflicts of interest.
Figures





References
-
- Assaraf Y.G., Brozovic A., Gonçalves A.C., Jurkovicova D., Linē A., Machuqueiro M., Saponara S., Sarmento-Ribeiro A.B., Xavier C.P.R., Vasconcelos M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates. 2019;46:100645. doi: 10.1016/j.drup.2019.100645. - DOI - PubMed
-
- da Silveira Júnior L.S., Soares V.L., da Silva A.S.J., Gil E.A., Araújo M.d.G.P.d., Gonçalves C.A.M., Paiva A.d.S., de Oliveira T.M.M., Oliveira G.H.d.M., e Silva D.G.K.C., et al. P-glycoprotein and multidrug resistance-associated protein-1 expression in acute myeloid leukemia: Biological and prognosis implications. Int. J. Lab. Hematol. 2020;42:594–603. doi: 10.1111/ijlh.13241. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

