Safety of Inclisiran: A Disproportionality Analysis from the EudraVigilance Database
- PMID: 39459005
- PMCID: PMC11511047
- DOI: 10.3390/ph17101365
Safety of Inclisiran: A Disproportionality Analysis from the EudraVigilance Database
Abstract
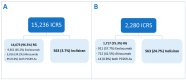
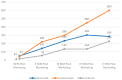
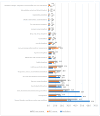
Introduction: The discovery of serine protease proprotein convertase subtilisin-kexin type 9 (PCSK9) has revolutionized pharmacological lipid-lowering treatments. The first PCSK9 antagonists (PCSK9-A), evolocumab and alirocumab, were approved in 2015. Targeting PCSK9 synthesis marked a major advancement in this field, leading to the development of inclisiran, a long-acting siRNA targeting PCSK9 mRNA. However, real-world safety data on this drug are still limited. Therefore, this study aims to provide a real-world safety evaluation of inclisiran, comparing its characteristics to those of PCSK9-As. Methods: A retrospective pharmacovigilance study was conducted using EudraVigilance (EV). Inclisiran-related individual case safety reports (I-ICSRs) from 01/01/2021 to 06/30/2023 were retrieved. ICSRs for evolocumab or alirocumab from 01/01/2015 to 06/30/2023 were collected as a reference group (RG). ADRs were classified using the MedDRA dictionary. Data were evaluated using descriptive and disproportionality analyses. Crude reporting odds ratio (ROR) with 95% confidence intervals (CI) were used as disproportionality measures. Results: Of the 15,236 ICSRs, 3.7% (n = 563) involved inclisiran, with the rest in the RG. Most I-ICSRs involved female patients (51.7%) aged 18 to 64 (52.8%). The most-reported ADRs for inclisiran were "general disorders and administration site conditions" (n = 347) and "investigations" (n = 277). Significant disproportionality was found in I-ICSRs compared to the RG for "Myalgia" (ROR: 2.43; 95% CI: 1.94-3.04), "Low-density lipoprotein increased" (ROR: 11.95; 95% CI: 9.10-15.52), and "Drug ineffective" (ROR: 6.37; 95% CI: 4.64-8.74). Conclusions: The inclisiran safety profile aligns with the existing literature and pre-commercial data. However, further studies are needed to fully understand the observed differences with PCSK9-As.
Keywords: PCSK9 antagonist; adverse drug reactions; alirocumab; evolocumab; inclisiran; pharmacovigilance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
-
- Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:625. doi: 10.1161/CIR.0000000000000625. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous