Evaluation of Safety and Efficacy of Cell Therapy Based on Osteoblasts Derived from Umbilical Cord Mesenchymal Stem Cells for Osteonecrosis of the Femoral Head: Study Protocol for a Single-Center, Open-Label, Phase I Clinical Trial
- PMID: 39459006
- PMCID: PMC11510171
- DOI: 10.3390/ph17101366
Evaluation of Safety and Efficacy of Cell Therapy Based on Osteoblasts Derived from Umbilical Cord Mesenchymal Stem Cells for Osteonecrosis of the Femoral Head: Study Protocol for a Single-Center, Open-Label, Phase I Clinical Trial
Abstract
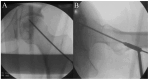
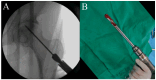
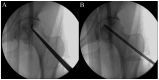
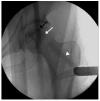
Although mesenchymal stem cells (MSCs) insertion has gained recent attention as a joint-preserving procedure, no study has conducted direct intralesional implantation of human umbilical cord-derived MSCs (hUCMSCs) in patients with ONFH. This is a protocol for a phase 1 clinical trial designed to assess the safety and exploratory efficacy of human umbilical cord-derived osteoblasts (hUC-Os), osteogenic differentiation-induced cells from hUCMSCs, in patients with early-stage ONFH. Nine patients with Association Research Circulation Osseous (ARCO) stage 1 or 2 will be assigned to a low-dose (1 × 107 hUC-O cells, n = 3), medium-dose (2 × 107 cells, n = 3), and high-dose group (4 × 107 cells, n = 3) in the order of their arrival at the facility, and, depending on the occurrence of dose-limiting toxicity, up to 18 patients can be enrolled by applying the 3 + 3 escalation method. We will perform hUC-O (CF-M801) transplantation combined with core decompression and follow-up for 12 weeks according to the study protocol. Safety will be determined through adverse event assessment, laboratory tests including a panel reactive antibody test, vital sign assessment, physical examination, and electrocardiogram. Efficacy will be explored through the change in pain visual analog scale, Harris hip score, Western Ontario and McMaster Universities Osteoarthritis Index, ARCO stage, and also size and location of necrotic lesion according to Japanese Investigation Committee classification before and after the procedure. Joint preservation is important, particularly in younger, active patients with ONFH. Confirmation of the safety and efficacy of hUC-Os will lead to a further strategy to preserve joints for those suffering from ONFH and improve our current knowledge of cell therapy.
Keywords: CF-M801; cell therapy; core decompression; hip; osteoblast; osteonecrosis of the femoral head; umbilical cord-derived mesenchymal stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

