Cobaltabis(Dicarbollide) [ o-COSAN]- for Boron Neutron Capture Therapy of Head and Neck Cancer: Biodistribution and Irradiation Studies in an Experimental Oral Cancer Model
- PMID: 39459007
- PMCID: PMC11510372
- DOI: 10.3390/ph17101367
Cobaltabis(Dicarbollide) [ o-COSAN]- for Boron Neutron Capture Therapy of Head and Neck Cancer: Biodistribution and Irradiation Studies in an Experimental Oral Cancer Model
Abstract
Background: Boron neutron capture therapy (BNCT) is a tumor-selective particle radiotherapy that combines preferential boron accumulation in tumors and neutron irradiation. Based on previous studies in tumor-bearing mice, this study evaluated the biodistribution of the sodium salt of cobaltabis(dicarbollide) (Na[3,3'-Co(C2B9H11)2], abbreviated as Na[o-COSAN]) in the hamster cheek pouch oral cancer model and the Na[o-COSAN]/BNCT therapeutic effect on tumors and induced radiotoxicity. The synthesis and comprehensive characterization of 10B-enriched trimethylammonium salt of nido-[7,8-C210B9H12]-o-carborane, along with the cesium and sodium salts of [o-10COSAN] cobaltabis(dicarbollide) are reported here for the first time.
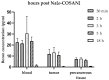
Methods: Hamsters bearing tumors were injected with Na[o-COSAN] (7.5 mg B/kg) and euthanized at different time-points after injection (30 min, 2, 3, 5, and 18 h post-administration) to evaluate boron uptake in different tissues/organs. Based on these results, tumor-bearing animals were treated with Na[10B-o-COSAN]/BNCT (7.5 mg B/kg b.w., 3 h), prescribing 5 Gy total in absorbed dose to the precancerous tissue surrounding tumors, i.e., the dose-limiting tissue.
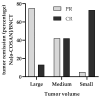
Results: Na[o-10COSAN] exhibited no toxicity. Although biodistribution studies employing Na[o-COSAN] have shown low absolute boron concentration in the tumor (approx. 11 ppm), Na[o-10COSAN]/BNCT induced a high and significant therapeutic effect on tumors versus the control group (cancerized, untreated animals). Moreover, only half of the animals exhibited severe mucositis in the precancerous dose-limiting tissue after BNCT, which resolved completely at 21 days after irradiation.
Conclusions: Na[o-10COSAN] would be potentially useful to treat head and neck cancer with BNCT.
Keywords: BNCT; Na[o-COSAN]; biodistribution studies; boron carrier; boron neutron capture therapy; hamster; head and neck cancer; metallacarborane.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

