Exome Sequence Data of Eight SLC Transporters Reveal That SLC22A1 and SLC22A3 Variants Alter Metformin Pharmacokinetics and Glycemic Control
- PMID: 39459024
- PMCID: PMC11510168
- DOI: 10.3390/ph17101385
Exome Sequence Data of Eight SLC Transporters Reveal That SLC22A1 and SLC22A3 Variants Alter Metformin Pharmacokinetics and Glycemic Control
Abstract
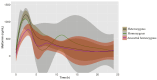
Background: Type 2 diabetes (T2D) is one of the leading causes of mortality and is a public health challenge worldwide. Metformin is the first-choice treatment for T2D; its pharmacokinetics (PK) is facilitated by members of the solute carrier (SLC) superfamily of transporters, it is not metabolized, and it is excreted by the kidney. Although interindividual variability in metformin pharmacokinetics is documented in the Mexican population, its pharmacogenomics is still underexplored. We aimed to identify variants in metformin SLC transporter genes associated with metformin PK and response in Mexican patients. Methods: Using exome data from 2217 Mexican adults, we identified 86 biallelic SNVs in the eight known genes encoding SLC transporters, with a minor allele frequency ≥ 1%, which were analyzed in an inadequate glycemic control (IGC) association study in T2D metformin treated patients. Metformin PK was evaluated in a pediatric cohort and the effect of associated SNVs was correlated. Results: Functional annotation classified two SNVs as pathogenic. The association study revealed two blocks associated with IGC. These haplotypes comprise rs622591, rs4646272, rs4646273, and rs4646276 in SLC22A1; and rs1810126 and rs668871 in SLC22A3. PK profiles revealed that homozygotes of the SLC22A1 haplotype reached lower plasma metformin concentrations 2 h post administration than the other groups. Conclusions: Our findings highlight the potential of pharmacogenomics studies to enhance precision medicine, which may involve dosage adjustments or the exploration of alternative therapeutic options. These hold significant implications for public health, particularly in populations with a high susceptibility to develop metabolic diseases, such as Latin Americans.
Keywords: SLC transporters; SNVs; metformin response; pharmacogenetics; pharmacokinetics; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- PAHO_WHO Diabetes. Pan American Health Organization. [(accessed on 1 October 2024)]. Available online: https://www.paho.org/en/topics/diabetes#:~:text=Diabetes%20is%20a%20majo....
-
- World Health Organization Diabetes Fact Sheet. [(accessed on 6 February 2023)]; Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
-
- Romero R., Erez O., Hüttemann M., Maymon E., Panaitescu B., Conde-Agudelo A., Pacora P., Yoon B.H., Grossman L.I. Metformin, the Aspirin of the 21st Century: Its Role in Gestational Diabetes Mellitus, Prevention of Preeclampsia and Cancer, and the Promotion of Longevity. Am. J. Obstet. Gynecol. 2017;217:282–302. doi: 10.1016/j.ajog.2017.06.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

