Hydroxyurea Pharmacokinetic Evaluation in Patients with Sickle Cell Disease
- PMID: 39459025
- PMCID: PMC11510670
- DOI: 10.3390/ph17101386
Hydroxyurea Pharmacokinetic Evaluation in Patients with Sickle Cell Disease
Abstract
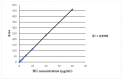
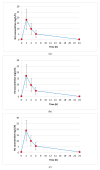
Background: Hydroxyurea (HU), also known as hydroxycarbamide, is an oral ribonucleotide reductase inhibitor. In 1999, the United States Food and Drug Administration (FDA) approved HU for the treatment of sickle cell disease (SCD). Since then, it has become the cornerstone in the management of SCD patients, helping to reduce vaso-occlusive crises, acute chest syndrome, the need for blood transfusions, hospitalizations and mortality. There is considerable variability among individuals in HU pharmacokinetic (Pk) parameters that can influence treatment efficacy and toxicity. The objective of this work is part of a clinical study aimed at investigating HU Pk and determining the optimal sampling time to estimate the Area Under the Curve (AUC) in SCD patients. Methods: HU plasma concentration in 80 patients at various time points (2, 4, 6, 24 h) following a 48-h drug washout was quantified using High-Pressure Liquid Chromatography (HPLC) coupled with an ultraviolet (UV) detection method previously described in the literature and adapted to new conditions with partial modifications. Results: The mean HU administered dose was 19.5 ± 5.1 mg/kg (range: 7.7-37.5 mg/kg). The median AUC quantified in plasma patients was 101.3 mg/L/h (Interquartile Range (IQR): 72.5-355.9) and it was not influenced by the weight-based dose. However, there was a strong positive correlation between AUC and Body Mass Index (BMI) as well as dose per Body Surface Area (BSA). Along with a three-point approach for AUC determination present in the literature, we show results obtained from a four-point sampling strategy, which is more useful and effective for better optimizing dose escalation to the maximum tolerated dose (MTD). Moreover, we observed that most patients achieved the maximum HU plasma concentration two hours after drug administration, regardless of age differences. Conclusions: HU treatment, which represents a milestone in the treatment of SCD due to its ability to reduce disease complications and improve patients' quality of life, requires careful monitoring to optimize the individual dose for saving potential side effects and/or adverse events.
Keywords: area under the curve; high-pressure liquid chromatography; maximum tolerated dose; sex; therapeutic drug monitoring; variability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Development of a pharmacokinetic-guided dose individualization strategy for hydroxyurea treatment in children with sickle cell anaemia.Br J Clin Pharmacol. 2016 Apr;81(4):742-52. doi: 10.1111/bcp.12851. Epub 2016 Feb 5. Br J Clin Pharmacol. 2016. PMID: 26615061 Free PMC article.
-
Plasma and urine hydroxyurea levels might be useful in the management of adult sickle cell disease.Hemoglobin. 2007;31(4):417-25. doi: 10.1080/03630260701587745. Hemoglobin. 2007. PMID: 17994375
-
Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive "switching" agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia.Medicine (Baltimore). 1996 Nov;75(6):300-26. doi: 10.1097/00005792-199611000-00002. Medicine (Baltimore). 1996. PMID: 8982148 Clinical Trial.
-
Hydroxyurea for the treatment of sickle cell disease.Evid Rep Technol Assess (Full Rep). 2008 Mar;(165):1-95. Evid Rep Technol Assess (Full Rep). 2008. PMID: 18457478 Free PMC article. Review.
-
A systematic review on hydroxyurea therapy for sickle cell disease in India.Indian J Med Res. 2022 Aug;156(2):299-311. doi: 10.4103/ijmr.ijmr_3447_21. Indian J Med Res. 2022. PMID: 36629190 Free PMC article.
References
-
- Mañú Pereira M.D.M., Colombatti R., Alvarez F., Bartolucci P., Bento C., Brunetta A.L., Cela E., Christou S., Collado A., de Montalembert M., et al. Sickle cell disease landscape and challenges in the EU: The ERN-EuroBloodNet perspective. Lancet Haematol. 2023;10:e687–e694. doi: 10.1016/S2352-3026(23)00182-5. - DOI - PubMed
-
- Thomson N.J.K., Teply C., Lonberg N., Vilchis Tella V.M. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Hematol. 2023;10:e585–e599. doi: 10.1016/S2352-3026(23)00118-7. - DOI - PMC - PubMed
-
- De Franceschi L., Castiglioni C., Condorelli C., Valsecchi D., Premoli E., Fiocchi C., Perrone V., Esposti L.D., Forni G.L. On Behalf of The GREATalyS Study Group. Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy. J. Clin. Med. 2022;12:117. doi: 10.3390/jcm12010117. - DOI - PMC - PubMed
-
- Sheehan V.A., Gordeuk V.R., Kutlar A. Williams Hematology. 10th ed. McGraw-Hill Education; New York, NY, USA: 2021. Disorders of Hemoglobin Structure: Sickle Cell Anemia and Related Abnormalities.
LinkOut - more resources
Full Text Sources

