Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications
- PMID: 39459028
- PMCID: PMC11510357
- DOI: 10.3390/ph17101389
Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications
Abstract
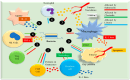
Hypoxia is a crucial factor in tumor biology, affecting various solid tumors to different extents. Its influence spans both early and advanced stages of cancer, altering cellular functions and promoting resistance to therapy. Hypoxia reduces the effectiveness of radiotherapy, chemotherapy, and immunotherapy, making it a target for improving therapeutic outcomes. Despite extensive research, gaps persist, necessitating the exploration of new chemical and pharmacological interventions to modulate hypoxia-related pathways. This review discusses the complex pathways involved in hypoxia and the associated pharmacotherapies, highlighting the limitations of current treatments. It emphasizes the potential of nanoparticle-based platforms for delivering anti-hypoxic agents, particularly oxygen (O2), to the tumor microenvironment. Combining anti-hypoxic drugs with conventional cancer therapies shows promise in enhancing remission rates. The intricate relationship between hypoxia and tumor progression necessitates novel therapeutic strategies. Nanoparticle-based delivery systems can significantly improve cancer treatment efficacy by targeting hypoxia-associated pathways. The synergistic effects of combined therapies underscore the importance of multimodal approaches in overcoming hypoxia-mediated resistance. Continued research and innovation in this area hold great potential for advancing cancer therapy and improving patient outcomes.
Keywords: cancer therapy; hypoxia; nanoparticle delivery; therapy resistance; tumor biology.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the information described in the manuscript. This includes employment, consultancies, honoraria, expert testimonies, patents received, or royalties.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

