Auranofin as a Novel Anticancer Drug for Anaplastic Thyroid Cancer
- PMID: 39459033
- PMCID: PMC11510098
- DOI: 10.3390/ph17101394
Auranofin as a Novel Anticancer Drug for Anaplastic Thyroid Cancer
Abstract
Background/Objectives: Anaplastic thyroid cancer (ATC) is an aggressive and rare cancer with a poor prognosis, and traditional therapies have limited efficacy. This study investigates drug repositioning, focusing on auranofin, a gold-based drug originally used for rheumatoid arthritis, as a potential treatment for ATC. Methods: Auranofin was identified from an FDA-approved drug library and tested on two thyroid cancer cell lines, 8505C and FRO. Antitumor efficacy was evaluated through gene and protein expression analysis using Western blot, FACS, and mRNA sequencing. In vivo experiments were conducted using subcutaneous injections in nude mice to confirm the anticancer effects of auranofin. Results: Auranofin induced reactive oxygen species (ROS) production and apoptosis, leading to a dose-dependent reduction in cell viability, G1/S phase cell cycle arrest, and altered expression of regulatory proteins. It also inhibited cancer stem cell activity and suppressed epithelial-mesenchymal transition. mRNA sequencing revealed significant changes in the extracellular matrix-receptor interaction pathway, supported by Western blot results. In vivo xenograft models demonstrated strong antitumor activity. Conclusions: Auranofin shows promise as a repurposed therapeutic agent for ATC, effectively inhibiting cell proliferation, reducing metastasis, and promoting apoptosis. These findings suggest that auranofin could play a key role in future ATC treatment strategies.
Keywords: anaplastic thyroid cancer; auranofin; chemotherapy; drug repositioning.
Conflict of interest statement
Authors Soonchul Lee and Hyun-Ju An were employed by the company SL Bio, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




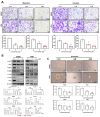
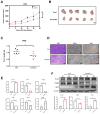
References
-
- Limaiem F., Kashyap S., Naing P.T., Giwa A.O. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Anaplastic Thyroid Cancer. - PubMed
-
- Graceffa G., Salamone G., Contino S., Saputo F., Corigliano A., Melfa G., Proclamà M.P., Richiusa P., Mazzola S., Tutino R., et al. Risk Factors for Anaplastic Thyroid Carcinoma: A Case Series From a Tertiary Referral Center for Thyroid Surgery and Literature Analysis. Front. Oncol. 2022;12:948033. doi: 10.3389/fonc.2022.948033. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

