Cyclization Strategies in Carbonyl-Olefin Metathesis: An Up-to-Date Review
- PMID: 39459236
- PMCID: PMC11510574
- DOI: 10.3390/molecules29204861
Cyclization Strategies in Carbonyl-Olefin Metathesis: An Up-to-Date Review
Abstract
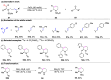
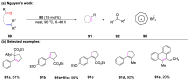
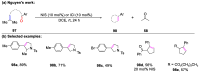
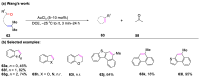
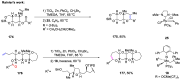
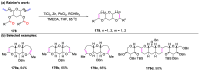
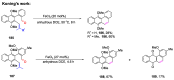
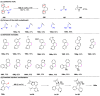
The metathesis reaction between carbonyl compounds and olefins has emerged as a potent strategy for facilitating swift functional group interconversion and the construction of intricate organic structures through the creation of novel carbon-carbon double bonds. To date, significant progress has been made in carbonyl-olefin metathesis reactions, where oxetane, pyrazolidine, 1,3-diol, and metal alkylidene have been proved to be key intermediates. Recently, several reviews have been disclosed, focusing on distinct catalytic approaches for achieving carbonyl-olefin metathesis. However, the summarization of cyclization strategies for constructing aromatic heterocyclic frameworks through carbonyl-olefin metathesis reactions has rarely been reported. Consequently, we present an up-to-date review of the cyclization strategies in carbonyl-olefin metathesis, categorizing them into three main groups: the formation of monocyclic compounds, bicyclic compounds, and polycyclic compounds. This review delves into the underlying mechanism, scope, and applications, offering a comprehensive perspective on the current strength and the limitation of this field.
Keywords: bicyclic compounds; carbonyl–olefin metathesis; cyclization; monocyclic compounds; polycyclic compounds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











































References
-
- Wang S., Zhang Y.L., Ba Y.Y., Zhang J.Y., Sun D.M. Study and Applications of Stereoselective Olefin Metathesis Reactions. Chin. J. Org. Chem. 2020;40:2725–2741. doi: 10.6023/cjoc202003054. - DOI
-
- Liu P., Ai C.J. Olefin Metathesis Reaction in Rubber Chemistry and Industry and Beyond. Ind. Eng. Chem. Res. 2018;57:3807–3820. doi: 10.1021/acs.iecr.7b03830. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

