Serendipitous Conversion of an Acetylamino Dideoxy-Octonic Acid Derivate into a Functionalized Carbohydrate-Pyrazole Conjugate and Investigation of the Method´s General Applicability
- PMID: 39459253
- PMCID: PMC11509885
- DOI: 10.3390/molecules29204885
Serendipitous Conversion of an Acetylamino Dideoxy-Octonic Acid Derivate into a Functionalized Carbohydrate-Pyrazole Conjugate and Investigation of the Method´s General Applicability
Abstract
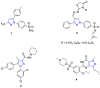
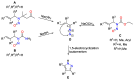
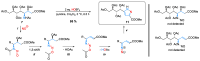
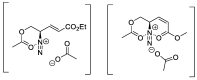
By treatment of the peracetylated methylester of 4-acetylamino-2,4-dideoxy-d-glycero-d-galacto-octonic acid (ADOA-PAE) with nitrosyl tetrafluoroborate, a serendipitous formation of a highly functionalized carbohydrate-pyrazole conjugate was observed in 95% yield. This observation is remarkable, as it involves a five-step one-pot synthesis that proceeds via an 1,3-acyl shift and a 1,5-electrocyclization, which usually requires thermal conditions; however, the reaction occurred at a temperature of 0 °C. Additionally, the excellent yield of the carbohydrate-decorated pyrazole and the regiospecificity of the cyclization are of particular interest, as regioselectivity is always a challenge in pyrazole synthesis. Subsequently, this novel access to pyrazoles starting from N-acetyl-allyl amides via nitrosation and electrocyclization was investigated. In addition, mechanistic studies for the formation of substituted pyrazoles of type were carried out.
Keywords: ADOA; pyrazole synthesis; sialic acid derivate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Trofimenko S. The Coordination Chemistry of Pyrazole-Derived Ligands. Chem. Rev. 1972;72:497–509. doi: 10.1021/cr60279a003. - DOI
LinkOut - more resources
Full Text Sources

