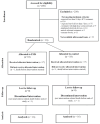
Functional Magnetic Neuromuscular Stimulation vs. Routine Physiotherapy in the Critically Ill for Prevention of ICU Acquired Muscle Loss: A Randomised Controlled Trial
- PMID: 39459511
- PMCID: PMC11509331
- DOI: 10.3390/medicina60101724
Functional Magnetic Neuromuscular Stimulation vs. Routine Physiotherapy in the Critically Ill for Prevention of ICU Acquired Muscle Loss: A Randomised Controlled Trial
Abstract
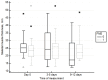
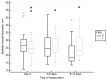
Background and Objectives: Muscle loss is a known complication of ICU admission. The aim of the study was to investigate the effect of neuromuscular functional magnetic stimulation (FMS) on quadriceps muscle thickness in critically ill patients. Materials and Methods: Among ICU patients one quadriceps was randomized to FMS (Tesla Stym, Iskra Medical, Ljubljana, Slovenia) stimulation and the other to control care. Quadriceps thickness was measured by ultrasound (US) in transversal and longitudinal planes at enrolment, Days 3-5, and Days 9-12. The trial stopped early following an interim analysis comparing muscle thickness differences between groups using repeated measures ANOVA. Results: Of 18 patients randomized, 2 died before completing the trial. The final analysis reported included 16 patients (female 38%, age 68 ± 10 years, SOFA 10.8 ± 2.7). Three mild skin thermal injuries were noted initially, which were later avoided with proper positioning of FMS probe. Primary outcome comparison showed that quadriceps thickness in transversal and longitudinal planes decreased in the non-stimulated legs and, but it did not change in FMS legs (-4.1 mm (95%CI: -9.4 to -0.6) vs. -0.7 mm (95%CI: -4.1 to -0.7) (p = 0.03) and -4.4 mm (95%CI: -8.9 to -1.1) vs. -1.5 mm (95%CI: -2.6 to -2.2) (p = 0.02), respectively) (ANOVA difference between groups p = 0.036 and 0.01, respectively). Conclusions: In the critically ill, neuromuscular FMS is feasible and safe with precautions applied to avoid possible skin thermal injury. FMS decreases the loss of quadriceps muscle thickness.
Keywords: ICU acquired weakness; functional magnetic stimulation; muscle ultrasound; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous