Palmitoylethanolamide (PEA) for Prevention of Gastroesophageal Inflammation: Insights from In Vitro Models
- PMID: 39459521
- PMCID: PMC11508466
- DOI: 10.3390/life14101221
Palmitoylethanolamide (PEA) for Prevention of Gastroesophageal Inflammation: Insights from In Vitro Models
Abstract
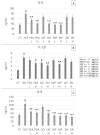
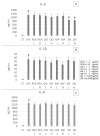
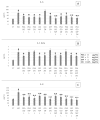
Gastroesophageal reflux disease (GERD) is a digestive disorder that can lead to chronic mucosal damage, causing esophagitis, Barrett's esophagus and esophageal cancer. GERD currently affects about 13% of the world's population and represent a major public health concern due to the increasing prevalence and incidence. The aim of this study was to explore complementary strategies for GERD management based the natural compound palmitoylethanolamide (PEA), alone or associated with plant extracts with demonstrated anti-GERD activity (Zingiber officinale, Musa × paradisiaca, Opuntia ficus-indica and Olea europaea). For this purpose, two in vitro models based on the esophageal mucosa CP-B cell line were chosen. The first one was based on the exposure of esophageal cells to HCl, while the second one was based on lipopolysaccharide (LPS) treatment to cause a strong inflammatory cell response. Inflammation induced was assessed using a Luminex® assay, measuring the secretion of IL-1β, IL-6, IL-10, IL-8 and TNF-α. Results obtained demonstrate that PEA strongly decreased the inflammatory response elicited by HCl exposure. Moreover, the effect of PEA was enhanced by the presence of natural extracts of Zingiber officinale, Musa × paradisiaca, Opuntia ficus-indica and Olea europaea. PEA should be considered as an anti-GERD natural compound of interest.
Keywords: Musa × paradisiaca; Olea europaea; Opuntia ficus-indica; Zingiber officinale; gastroesophageal reflux disease (GERD); palmitoylethanolamide (PEA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

