The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome-A Comprehensive Review
- PMID: 39459527
- PMCID: PMC11508959
- DOI: 10.3390/life14101227
The Gut-Brain Axis in Opioid Use Disorder: Exploring the Bidirectional Influence of Opioids and the Gut Microbiome-A Comprehensive Review
Abstract
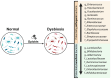
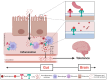
Opioid Use Disorder is a chronic condition characterized by compulsive opioid use despite negative consequences, resulting in severe health risks such as overdose and contraction of infectious diseases. High dropout rates in opioid agonist therapy highlight the need for more effective relapse prevention strategies. Animal and clinical studies indicate that opioids influence gut microbiota, which in turn plays a critical role in addiction development and alters behavioral responses to opioids. This study provides a comprehensive review of the literature on the effects of opioids on the gut microbiome and explores the potential of microbiome manipulation as a therapeutic target in opioid addiction.
Keywords: addiction; gut-brain axis; microbiome; microbiota; opioid use disorder; opioids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

