The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review
- PMID: 39459534
- PMCID: PMC11508655
- DOI: 10.3390/life14101234
The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review
Abstract
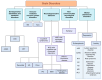
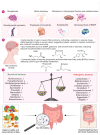
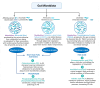
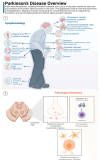
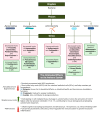
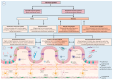
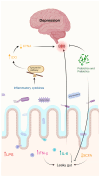
Microbes have inhabited the earth for hundreds of millions of years longer than humans. The microbiota-gut-brain axis (MGBA) represents a bidirectional communication pathway. These communications occur between the central nervous system (CNS), the enteric nervous system (ENS), and the emotional and cognitive centres of the brain. The field of research on the gut-brain axis has grown significantly during the past two decades. Signalling occurs between the gut microbiota and the brain through the neural, endocrine, immune, and humoral pathways. A substantial body of evidence indicates that the MGBA plays a pivotal role in various neurological diseases. These include Alzheimer's disease (AD), autism spectrum disorder (ASD), Rett syndrome, attention deficit hyperactivity disorder (ADHD), non-Alzheimer's neurodegeneration and dementias, fronto-temporal lobe dementia (FTLD), Wilson-Konovalov disease (WD), multisystem atrophy (MSA), Huntington's chorea (HC), Parkinson's disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), temporal lobe epilepsy (TLE), depression, and schizophrenia (SCZ). Furthermore, the bidirectional correlation between therapeutics and the gut-brain axis will be discussed. Conversely, the mood of delivery, exercise, psychotropic agents, stress, and neurologic drugs can influence the MGBA. By understanding the MGBA, it may be possible to facilitate research into microbial-based interventions and therapeutic strategies for neurological diseases.
Keywords: microbiota; microbiota–gut–brain axis; neurological disorders and neurodegenerative diseases; psychotropic agents.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

