Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice
- PMID: 39459546
- PMCID: PMC11508561
- DOI: 10.3390/life14101246
Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice
Abstract
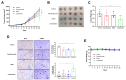
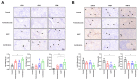
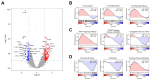
Bojungikki-Tang (BJIKT) is traditionally used to enhance digestive function and immunity. It has gained attention as a supplement to chemotherapy or targeted therapy owing to its immune-boosting properties. This study aimed to evaluate the synergistic anti-tumor effects of BJIKT in combination with pembrolizumab in a preclinical model. MHC I/II double knockout NSG mice were humanized with peripheral blood mononuclear cells (PBMCs) and injected subcutaneously with H460 lung tumor cells to establish a humanized tumor model. Both agents were administered to evaluate their impact on tumor growth and immune cell behavior. Immunohistochemistry showed decreased exhaustion markers in CD8(+) and CD4(+) T cells within the tumor, indicating enhanced T cell activity. Additionally, RNA sequencing, transcriptome analysis, and quantitative PCR analysis were performed on tumor tissues to investigate the molecular mechanisms underlying the observed effects. The results confirmed that BJIKT improved T cell function and tumor necrosis factor signaling while suppressing transforming growth factor-β signaling. This modulation led to cell cycle arrest and apoptosis. These findings demonstrate that BJIKT, when combined with pembrolizumab, produces significant anti-tumor effects by altering immune pathways and enhancing the anti-tumor immune response. This study provides valuable insights into the role of BJIKT in the tumor microenvironment and its potential to improve therapeutic outcomes.
Keywords: Bojungikki-Tang; anti-tumor; humanized mouse model; immunoregulation; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Edahiro Y., Koike M., Nojiri S., Harada Y., Gotoh A., Fujibayashi K., Nishizaki Y., Yanagisawa N., Takaku T., Nitta H., et al. A pilot study examining the efficacy of hochuekkito for improving quality of life in patients with myeloproliferative neoplasms. Jpn. J. Clin. Oncol. 2022;52:880–886. doi: 10.1093/jjco/hyac076. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

