Umbilical Cord Blood Platelet Lysate Eyedrops for the Treatment of Severe Ocular Surface Disorders in Graft vs. Host Disease Patients: Clinical Study
- PMID: 39459568
- PMCID: PMC11509496
- DOI: 10.3390/life14101268
Umbilical Cord Blood Platelet Lysate Eyedrops for the Treatment of Severe Ocular Surface Disorders in Graft vs. Host Disease Patients: Clinical Study
Abstract
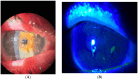
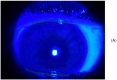
Background: Graft-versus-host disease (GvHD) is an overactive systemic inflammatory response that can arise following allogeneic hematopoietic stem cell transplantation (HSCT). This condition occurs when the transplanted donor immune cells recognize the recipient's tissues as foreign and trigger an immune response against them. The ocular surface (eyelids, conjunctiva, meibomian glands, lacrimal glands, and cornea) is particularly involved in GvHD, and its response to existing treatments, including potent immunosuppressants and new targeted therapies, is undesirable, with such treatments often being ineffective. Human allogeneic umbilical cord blood platelet lysate stands out as a potent adjunct to conventional therapies for ocular surface disorders related to severe Dry Eye Disease. This study aimed to evaluate the safety and efficacy of umbilical cord blood platelet lysate eyedrops for the treatment of severe ocular surface disorders in graft-versus-host disease patients who have received previous unsuccessful treatments. Methods: This study was a prospective, non-comparative, interventional case series study involving 22 patients (10 females and 12 males) aged 25-46 years with severe ocular surface disorders that were unresponsive to standard treatments. The GvHD patients were categorized based on the severity of their ocular surface disorders into three groups: Group I: five patients with severe Dry Eye Disease and filamentary keratitis; Group II: eight patients suffering from severe blepharo-kerato-epitheliopathy; Group III: nine patients with corneal ulcers. Fresh umbilical cord blood (UCB) was obtained from healthy donors and subjected to centrifugation using a novel PRP preparation kit provided by Sciacca (AG) Cord blood bank, Italy in a one-step process. In all groups, the outcomes before and after treatment were evaluated by means of the OSDI (Ocular Surface Disease Index), SANDE (Symptom Assessment in Dry Eye) questionnaire, VAS (Visual Analogue Scale), slit lamp examination, Esthesiometry, Lissamine Green Staining, the NIBUT (Non-Invasive Break-Up Time) and BUT, fluorescein staining with digital photography and Oxford classification, the Schirmer Test, the Best Corrected Visual Acuity (BCVA), and Meibography. In Group III at each evaluation time, the size of the ulcer and its relative reduction compared to the baseline size were recorded. Clinical variables, such as corneal inflammation, conjunctivalization, corneal neovascularization, or pain, were also considered individually. Results: We observed a significant improvement in the SANDE, VAS, and OSDI scores; Schirmer Test; BUT; BCVA; and Oxford classification after treatment with allogeneic cord blood serum eyedrops. Nevertheless, pain and inflammation reduced markedly over time until complete healing in all cases. The mean reduction in the ulcer surface area (compared to baseline values) was significantly higher at all assessment points (p = 0.001 for day 7 and p < 0.001 for subsequent time points every 30 days for 90 days). At the last check-up (after 90 days of treatment), the number of ulcers (Group III, nine patients) with a reduction in size of greater than 50% was eight (88.8%), of which seven ulcers were completely healed. None of the patients experienced treatment-related local or systemic adverse events. In this study, using a relatively large number of cases, we demonstrated that the use of umbilical cord blood platelet lysate eyedrops is a safe, feasible, and effective curative approach for severe ocular surface disease in patients with GvHD. Conclusions: Our pilot study highlights the remarkable effectiveness of allogeneic cord blood serum eyedrops in patients with severe ocular surface disorders following GvHD who have shown an inadequate response to the usual treatments. It is mandatory to design future studies on the efficacy of this therapeutic approach for acute ocular, mucosal, and cutaneous GvHD.
Keywords: autoimmunity; corneal ulcers; graft-versus-host disease; ocular surface disorders; umbilical cord blood serum.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Umbilical Cord Blood-Derived Products in Autoimmune Systemic Syndromes with Severe Dryness: A Pilot Study.Medicina (Kaunas). 2024 Oct 28;60(11):1764. doi: 10.3390/medicina60111764. Medicina (Kaunas). 2024. PMID: 39596949 Free PMC article.
-
The Application of Umbilical Cord Blood-derived Platelet Gel for Skin Ulcers Associated With Chronic Graft-Versus-Host Disease in Pediatrics: A Randomized Trial.Transplant Cell Ther. 2024 Jul;30(7):694.e1-694.e10. doi: 10.1016/j.jtct.2024.04.013. Epub 2024 Apr 24. Transplant Cell Ther. 2024. PMID: 38663767 Clinical Trial.
-
A Randomized Trial of Topical Fibrinogen-Depleted Human Platelet Lysate Treatment of Dry Eye Secondary to Chronic Graft-versus-Host Disease.Ophthalmol Sci. 2022 Jun 2;2(3):100176. doi: 10.1016/j.xops.2022.100176. eCollection 2022 Sep. Ophthalmol Sci. 2022. PMID: 36245754 Free PMC article.
-
Ocular surface system alterations in ocular graft-versus-host disease: all the pieces of the complex puzzle.Graefes Arch Clin Exp Ophthalmol. 2019 Jul;257(7):1341-1351. doi: 10.1007/s00417-019-04301-6. Epub 2019 Apr 3. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30944986 Review.
-
Use of Acellular Umbilical Cord-Derived Tissues in Corneal and Ocular Surface Diseases.Medicines (Basel). 2021 Feb 9;8(2):12. doi: 10.3390/medicines8020012. Medicines (Basel). 2021. PMID: 33572327 Free PMC article. Review.
Cited by
-
Real-World Outcomes of Allogeneic Immunosafe Plasma Rich in Growth Factors Eye Drops for Refractory Ocular Surface Diseases: A Prospective Observational Study.Ophthalmol Ther. 2025 Sep;14(9):2253-2281. doi: 10.1007/s40123-025-01211-1. Epub 2025 Jul 25. Ophthalmol Ther. 2025. PMID: 40715787
References
-
- McDonald G.B., Sandmaier B.M., Mielcarek M., Sorror M.L. Survival, non-relapse mortality, and recurrent malignancy after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2015;21:213–221.
-
- Jagasia M.H., Greinix H.T., Arora M., Williams K.M. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

