Graphite-Phosphate Composites: Structure and Voltammetric Investigations
- PMID: 39459703
- PMCID: PMC11509367
- DOI: 10.3390/ma17205000
Graphite-Phosphate Composites: Structure and Voltammetric Investigations
Abstract
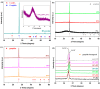
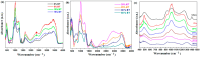
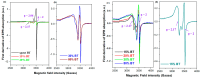
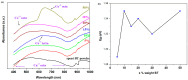
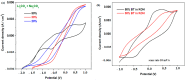
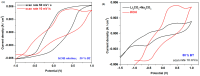
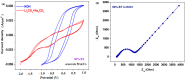
The utilization of lithium-ion batteries (LIBs) is increasing sharply with the increasing use of mobile phones, laptops, tablets, and electric vehicles worldwide. Technologies are required for the recycling and recovery of spent LIBs. In the context of the circular economy, it is urgent to search for new methods to recycle waste graphite that comes from the retired electrode of LIBs. The conversion of waste graphite into other products, such as new electrodes, in the field of energy devices is attractive because it reduces resource waste and processing costs, as well as preventing environmental pollution. In this paper, new electrode materials were prepared using waste anode graphite originating from a spent mobile phone battery with an xBT·0.1C12H22O11·(0.9-x)(NH4)2HPO4 composition, where x = 0-50 weight% BT from the anodic active mass of the spent phone battery (labeled as BT), using the melt quenching method. Analysis of the diffractograms shows the graphite crystalline phase with a hexagonal structure in all prepared samples. The particle sizes decrease by adding a higher BT amount in the composites. The average band gap is 1.32 eV (±0.3 eV). A higher disorder degree in the host network is the main factor responsible for lower band gap values. The prepared composites were tested as electrodes in an LIB or a fuel cell, achieving an excellent electrochemical performance. The voltammetric studies indicate that doping with 50% BT is the most suitable for applications as electrodes in LIBs and fuel cells.
Keywords: cyclic voltammetry; graphite composites; impedance; spent mobile phone battery; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ali H., Khan H.A., Pecht M.G. Circular economy of Li Batteries: Technologies and trends. J. Energy Storage. 2021;40:102690. doi: 10.1016/j.est.2021.102690. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

