COVID-19 in Relation to Polypharmacy and Immunization (2020-2024)
- PMID: 39459868
- PMCID: PMC11512247
- DOI: 10.3390/v16101533
COVID-19 in Relation to Polypharmacy and Immunization (2020-2024)
Abstract
Background: Observational studies reported worse COVID-19 evolution in relation to polypharmacy and reductions in COVID-19 hospital admissions and death in patients receiving chronic antihistamine treatment. The current profile of hospitalized patients with regard to different variants was analyzed to identify specific targets for future prospective trials.
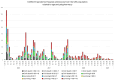
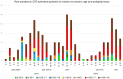
Methods: COVID-19 admissions to the Hospital of Terrassa (11 March 2020-28 August 2024 (n = 1457), from the integral Consorci Sanitari de Terrassa population (n = 167,386 people) were studied. Age, gender, the number of chronic treatments (nT), and immunization status were analyzed.
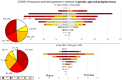
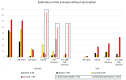
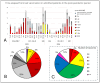
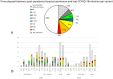
Results: After 5 May 2023, 291 patients (54% females) required COVID hospitalization. Of these, 39% received >8 nT (23% receiving 5-7 nT), 70.2% were >70 years, and 93.4% survived. In total, 12% of patients admitted after 5 May 2024 were not vaccinated, while 59% received ≥4 vaccines (43% within the last 12 months). In total, 49% of admitted patients presented no previous infection (while 3% presented infection during the last year). Delta or Omicron variants would have accounted for ≥80% of admissions > 60 years compared to the first pandemic wave if no vaccines existed.
Conclusions: Patients > 70 years who receive ≥5 nT, without prior COVID-19 infections, should be the priority for prevention, with updated vaccination and early treatments to reduce hospitalizations.
Trial registration: ClinicalTrials.gov NCT05504057.
Keywords: COVID-19; death rate; hospital admission; polypharmacy; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- University of Oxford-Oxford Martin School Official Data Collated by Our World in Data—Last Updated August 7th 2024—Processed by Our World in Data. [(accessed on 4 July 2024)]. Available online: https://ourworldindata.org/grapher/current-covid-hospitalizations-per-mi....
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

