Research Progress into the Biological Functions of IFITM3
- PMID: 39459876
- PMCID: PMC11512382
- DOI: 10.3390/v16101543
Research Progress into the Biological Functions of IFITM3
Abstract
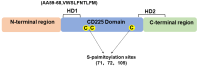
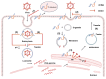
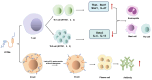
Interferon-induced transmembrane proteins (IFITMs) are upregulated by interferons. They are not only highly conserved in evolution but also structurally consistent and have almost identical structural domains and functional domains. They are all transmembrane proteins and have multiple heritable variations in genes. The IFITM protein family is closely related to a variety of biological functions, including antiviral immunity, tumor formation, bone metabolism, cell adhesion, differentiation, and intracellular signal transduction. The progress of the research on its structure and related functions, as represented by IFITM3, is reviewed.
Keywords: IFITM; IFITM3; function; structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L., et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7:e1001258. doi: 10.1371/journal.ppat.1001258. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

